Impaired cathepsin D in retinal pigment epithelium cells mediates Stargardt disease pathogenesis
- PMID: 38837708
- PMCID: PMC11296957
- DOI: 10.1096/fj.202400210RR
Impaired cathepsin D in retinal pigment epithelium cells mediates Stargardt disease pathogenesis
Abstract
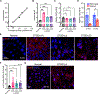
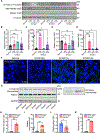
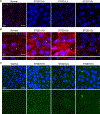
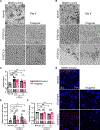
Recessive Stargardt disease (STGD1) is an inherited juvenile maculopathy caused by mutations in the ABCA4 gene, for which there is no suitable treatment. Loss of functional ABCA4 in the retinal pigment epithelium (RPE) alone, without contribution from photoreceptor cells, was shown to induce STGD1 pathology. Here, we identified cathepsin D (CatD), the primary RPE lysosomal protease, as a key molecular player contributing to endo-lysosomal dysfunction in STGD1 using a newly developed "disease-in-a-dish" RPE model from confirmed STGD1 patients. Induced pluripotent stem cell (iPSC)-derived RPE originating from three STGD1 patients exhibited elevated lysosomal pH, as previously reported in Abca4-/- mice. CatD protein maturation and activity were impaired in RPE from STGD1 patients and Abca4-/- mice. Consequently, STGD1 RPE cells have reduced photoreceptor outer segment degradation and abnormal accumulation of α-synuclein, the natural substrate of CatD. Furthermore, dysfunctional ABCA4 in STGD1 RPE cells results in intracellular accumulation of autofluorescent material and phosphatidylethanolamine (PE). The altered distribution of PE associated with the internal membranes of STGD1 RPE cells presumably compromises LC3-associated phagocytosis, contributing to delayed endo-lysosomal degradation activity. Drug-mediated re-acidification of lysosomes in the RPE of STGD1 restores CatD functional activity and reduces the accumulation of immature CatD protein loads. This preclinical study validates the contribution of CatD deficiencies to STGD1 pathology and provides evidence for an efficacious therapeutic approach targeting RPE cells. Our findings support a cell-autonomous RPE-driven pathology, informing future research aimed at targeting RPE cells to treat ABCA4-mediated retinopathies.
Keywords: cathepsin D; endo‐lysosome; phagocytosis; phosphatidylethanolamine; recessive Stargardt disease; retinal pigment epithelium.
© 2024 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of Interest Statement
ESYN, JH, and ZJ declare no conflicts of interest. Unrelated to this study, RAR served as a Principal Investigator for Sponsor Initiated Research Programs for: Eudora Therapeutics Inc., Biogen Inc., and NightStar Therapeutics. RAR served as a consultant for Replay Holdings, LLC; Oak Bay Biosciences, LTD; and Alexion Pharmaceuticals, Inc.
Figures


Similar articles
-
iPSC-derived retinal pigment epithelium: an in vitro platform to reproduce key cellular phenotypes and pathophysiology of retinal degenerative diseases.Stem Cells Transl Med. 2025 Mar 18;14(3):szae097. doi: 10.1093/stcltm/szae097. Stem Cells Transl Med. 2025. PMID: 39729520 Free PMC article. Review.
-
Expression of ABCA4 in the retinal pigment epithelium and its implications for Stargardt macular degeneration.Proc Natl Acad Sci U S A. 2018 Nov 20;115(47):E11120-E11127. doi: 10.1073/pnas.1802519115. Epub 2018 Nov 5. Proc Natl Acad Sci U S A. 2018. PMID: 30397118 Free PMC article.
-
Cell-autonomous lipid-handling defects in Stargardt iPSC-derived retinal pigment epithelium cells.Stem Cell Reports. 2022 Nov 8;17(11):2438-2450. doi: 10.1016/j.stemcr.2022.10.001. Epub 2022 Oct 27. Stem Cell Reports. 2022. PMID: 36306781 Free PMC article.
-
Complement modulation in the retinal pigment epithelium rescues photoreceptor degeneration in a mouse model of Stargardt disease.Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):3987-3992. doi: 10.1073/pnas.1620299114. Epub 2017 Mar 27. Proc Natl Acad Sci U S A. 2017. PMID: 28348233 Free PMC article.
-
Retina and RPE lipid profile changes linked with ABCA4 associated Stargardt's maculopathy.Pharmacol Ther. 2023 Sep;249:108482. doi: 10.1016/j.pharmthera.2023.108482. Epub 2023 Jun 27. Pharmacol Ther. 2023. PMID: 37385300 Free PMC article. Review.
Cited by
-
Aging of the eye: Lessons from cataracts and age-related macular degeneration.Ageing Res Rev. 2024 Aug;99:102407. doi: 10.1016/j.arr.2024.102407. Epub 2024 Jul 6. Ageing Res Rev. 2024. PMID: 38977082 Free PMC article. Review.
-
Whole RNA-Seq Analysis Reveals Longitudinal Proteostasis Network Responses to Photoreceptor Outer Segment Trafficking and Degradation in RPE Cells.Cells. 2025 Jul 29;14(15):1166. doi: 10.3390/cells14151166. Cells. 2025. PMID: 40801600 Free PMC article.
-
iPSC-derived retinal pigment epithelium: an in vitro platform to reproduce key cellular phenotypes and pathophysiology of retinal degenerative diseases.Stem Cells Transl Med. 2025 Mar 18;14(3):szae097. doi: 10.1093/stcltm/szae097. Stem Cells Transl Med. 2025. PMID: 39729520 Free PMC article. Review.
References
-
- Briggs CE, Rucinski D, Rosenfeld PJ, Hirose T, Berson EL, Dryja TP. Mutations in ABCR (ABCA4) in patients with Stargardt macular degeneration or cone-rod degeneration. Investigative ophthalmology & visual science. Sep 2001;42(10):2229–36. - PubMed
MeSH terms
Substances
Grants and funding
- R01 EY025002/EY/NEI NIH HHS/United States
- Research to Prevent Blindness (RPB)
- P30 EY000331/EY/NEI NIH HHS/United States
- UC | UCLA | Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, University of California Los Angeles (Broad Stem Cell Research Center)
- EY025002/HHS | NIH | National Eye Institute (NEI)
LinkOut - more resources
Full Text Sources
Miscellaneous

