Decreased Tiam1-mediated Rac1 activation is responsible for impaired directional persistence of chondrocyte migration in microtia
- PMID: 38837873
- PMCID: PMC11149491
- DOI: 10.1111/jcmm.18443
Decreased Tiam1-mediated Rac1 activation is responsible for impaired directional persistence of chondrocyte migration in microtia
Abstract
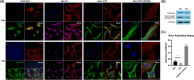
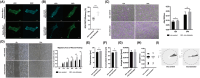
The human auricle has a complex structure, and microtia is a congenital malformation characterized by decreased size and loss of elaborate structure in the affected ear with a high incidence. Our previous studies suggest that inadequate cell migration is the primary cytological basis for the pathogenesis of microtia, however, the underlying mechanism is unclear. Here, we further demonstrate that microtia chondrocytes show a decreased directional persistence during cell migration. Directional persistence can define a leading edge associated with oriented movement, and any mistakes would affect cell function and tissue morphology. By the screening of motility-related genes and subsequent confirmations, active Rac1 (Rac1-GTP) is identified to be critical for the impaired directional persistence of microtia chondrocytes migration. Moreover, Rho guanine nucleotide exchange factors (GEFs) and Rho GTPase-activating proteins (GAPs) are detected, and overexpression of Tiam1 significantly upregulates the level of Rac1-GTP and improves directional migration in microtia chondrocytes. Consistently, decreased expression patterns of Tiam1 and active Rac1 are found in microtia mouse models, Bmp5se/J and Prkralear-3J/GrsrJ. Collectively, our results provide new insights into microtia development and therapeutic strategies of tissue engineering for microtia patients.
Keywords: Rac1‐GTP; Tiam1; chondrocytes; directional cell migration; microtia.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
-
Ankyrin-Tiam1 interaction promotes Rac1 signaling and metastatic breast tumor cell invasion and migration.J Cell Biol. 2000 Jul 10;150(1):177-91. doi: 10.1083/jcb.150.1.177. J Cell Biol. 2000. PMID: 10893266 Free PMC article.
-
Myoblast Migration and Directional Persistence Affected by Syndecan-4-Mediated Tiam-1 Expression and Distribution.Int J Mol Sci. 2020 Jan 27;21(3):823. doi: 10.3390/ijms21030823. Int J Mol Sci. 2020. PMID: 32012800 Free PMC article.
-
Tiam1 is recruited to β1-integrin complexes by 14-3-3ζ where it mediates integrin-induced Rac1 activation and motility.J Cell Physiol. 2011 Nov;226(11):2965-78. doi: 10.1002/jcp.22644. J Cell Physiol. 2011. PMID: 21302295 Free PMC article.
-
Mechanisms and consequences of dysregulation of the Tiam family of Rac activators in disease.Biochem Soc Trans. 2020 Dec 18;48(6):2703-2719. doi: 10.1042/BST20200481. Biochem Soc Trans. 2020. PMID: 33200195 Review.
Cited by
-
Systematic Review on Microtia: Current Knowledge and Future Directions.Children (Basel). 2025 Mar 25;12(4):411. doi: 10.3390/children12040411. Children (Basel). 2025. PMID: 40310042 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 2017PT32018/Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences
- 2018PT32015/Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences
- 32370884/National Natural Science Foundation of China
- 81871575/National Natural Science Foundation of China
- 2017-I2M-1-007/CAMS Innovation Fund for Medical Sciences
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

