Clinical Efficacy of Fuzheng Guben Anticancer Decoction Combined with Taxol in Treating Ovarian Carcinoma and Its Effect on Complication Incidence
- PMID: 38837981
- PMCID: PMC8709778
- DOI: 10.1155/2021/2782875
Clinical Efficacy of Fuzheng Guben Anticancer Decoction Combined with Taxol in Treating Ovarian Carcinoma and Its Effect on Complication Incidence
Abstract
Objective: To investigate the clinical value of Fuzheng Guben anticancer decoction combined with taxol in treating ovarian carcinoma (OC).
Methods: The medical records of 80 OC patients treated in the First People's Hospital of Fuyang Hangzhou (January 2018-January 2021) were retrospectively analyzed, and the patients were split into the control group and the experimental group according to the treatment regimen, with 40 cases each. Those in the control group accepted the taxol chemotherapy, and on this basis, those in the experimental group took the Fuzheng Guben anticancer decoction, so as to compare its clinical efficacy and complication incidence.
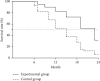
Results: No statistical between-group differences in patients' general information were observed (P > 0.05); compared with the control group, the disease objective remission rate of the experimental group was greatly higher (P < 0.05); before and after treatment, the changes in CD8+ were not significant, indicating no statistically significant between-group differences (P > 0.05), and after treatment, CD3+, CD4+, and CD4+/CD8+ were obviously higher than before and were obviously higher in the experimental group than in the control group (P < 0.05); after treatment, the CA125, CA199, and CEA levels were obviously lower than before and were significantly lower in the experimental group than in the control group (P < 0.05); the mean survival of the experimental group was significantly higher than that of the control group (19.80 ± 5.84 vs. 14.075 ± 5.12 months, P < 0.05); and between the two groups, the incidence rate of adverse reactions of the experimental group was remarkably lower (P < 0.05).
Conclusion: On the basis of taxol chemotherapy, jointly applying Fuzheng Guben anticancer decoction can significantly improve the clinical efficacy of OC, help to improve patients' immune function, lower the complication incidence rate, and prolong the mean survival.
Copyright © 2021 Pinger Li and Yinmei Lou.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Clinical Effect of Fuzheng Guben Decoction in the Treatment of Localized Prostate Cancer and Its Influence on Immune Function under Continuous Nursing Intervention.Contrast Media Mol Imaging. 2022 Sep 16;2022:3472722. doi: 10.1155/2022/3472722. eCollection 2022. Contrast Media Mol Imaging. 2022. Retraction in: Contrast Media Mol Imaging. 2023 Jul 19;2023:9820320. doi: 10.1155/2023/9820320. PMID: 36185576 Free PMC article. Retracted. Clinical Trial.
-
Clinical Efficacy of Bushen Yiqi Fuzheng Decoction Combined with Sunitinib in the Treatment of Postoperative Patients with Renal Cell Carcinoma and Its Influence on Their Immune Function.Arch Esp Urol. 2023 Sep;76(7):538-547. doi: 10.56434/j.arch.esp.urol.20237607.67. Arch Esp Urol. 2023. PMID: 37867340 Clinical Trial.
-
Efficacy of Modified Qingre Jiedu Decoction Combined with Three-Dimensional Conformal Radiotherapy in Treating Moderate to Advanced Ovarian Carcinoma and Its Effect on Levels of Serum Carcinoembryonic Antigen and Carbohydrate Antigen 125.Evid Based Complement Alternat Med. 2022 Jun 15;2022:1821719. doi: 10.1155/2022/1821719. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35754698 Free PMC article.
-
Effect of Fuzheng Yiliu decoction combined with chemotherapy on patients with intermediate and late stage gastrointestinal cancer.World J Gastroenterol. 2005 Jan 21;11(3):439-42. doi: 10.3748/wjg.v11.i3.439. World J Gastroenterol. 2005. PMID: 15637764 Free PMC article. Clinical Trial.
-
[Systematic review of Shenqi Fuzheng Injection combined with chemotherapy for treatment of breast cancer].Zhongguo Zhong Yao Za Zhi. 2019 Feb;44(3):589-596. doi: 10.19540/j.cnki.cjcmm.20180925.003. Zhongguo Zhong Yao Za Zhi. 2019. PMID: 30989927 Chinese.
References
-
- Matulonis U. A., Sill M. W., Makker V., et al. A randomized phase II study of cabozantinib versus weekly paclitaxel in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecologic Oncology . 2019;152(3):548–553. doi: 10.1016/j.ygyno.2018.12.008. - DOI - PMC - PubMed
-
- Yao H., Ma J. Dendrimer-paclitaxel complexes for efficient treatment in ovarian cancer: study on OVCAR-3 and HEK293T cells. Acta Biochimica Polonica . 2018;65(2):219–225. - PubMed
-
- Ye H., Liu X. J., Hui Y., Liang Y. H., Li C. H., Wan Q. USF1 gene polymorphisms may associate with the efficacy and safety of chemotherapy based on paclitaxel and prognosis in the treatment of ovarian cancer. Neoplasma: Journal of Experimental and Clinical Oncology . 2018;65(1):153–160. doi: 10.4149/neo_2018_170322n205. - DOI - PubMed
-
- Guo L., Zhang S., Chen C., Zeng H., Li F., Xu Q. Lentinan combined with cisplatin and paclitaxel in the treatment of patients with ovarian cancer with ascites. European Journal of Gynaecological Oncology . 2018;39(4):615–620.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

