Molecular screening and genetic diversity of tick-borne pathogens associated with dogs and livestock ticks in Egypt
- PMID: 38837987
- PMCID: PMC11152282
- DOI: 10.1371/journal.pntd.0012185
Molecular screening and genetic diversity of tick-borne pathogens associated with dogs and livestock ticks in Egypt
Abstract
Background: The Middle East and North Africa (MENA) offer optimal climatic conditions for tick reproduction and dispersal. Research on tick-borne pathogens in this region is scarce. Despite recent advances in the characterization and taxonomic explanation of various tick-borne illnesses affecting animals in Egypt, no comprehensive examination of TBP (tick-borne pathogen) statuses has been performed. Therefore, the present study aims to detect the prevalence of pathogens harbored by ticks in Egypt.
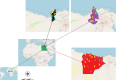
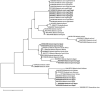
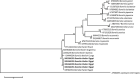
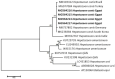
Methodology/principal findings: A four-year PCR-based study was conducted to detect a wide range of tick-borne pathogens (TBPs) harbored by three economically important tick species in Egypt. Approximately 86.7% (902/1,040) of the investigated Hyalomma dromedarii ticks from camels were found positive with Candidatus Anaplasma camelii (18.8%), Ehrlichia ruminantium (16.5%), Rickettsia africae (12.6%), Theileria annulata (11.9%), Mycoplasma arginini (9.9%), Borrelia burgdorferi (7.7%), Spiroplasma-like endosymbiont (4.0%), Hepatozoon canis (2.4%), Coxiella burnetii (1.6%) and Leishmania infantum (1.3%). Double co-infections were recorded in 3.0% (27/902) of Hy. dromedarii ticks, triple co-infections (simultaneous infection of the tick by three pathogen species) were found in 9.6% (87/902) of Hy. dromedarii ticks, whereas multiple co-infections (simultaneous infection of the tick by ≥ four pathogen species) comprised 12% (108/902). Out of 1,435 investigated Rhipicephalus rutilus ticks collected from dogs and sheep, 816 (56.9%) ticks harbored Babesia canis vogeli (17.1%), Rickettsia conorii (16.2%), Ehrlichia canis (15.4%), H. canis (13.6%), Bo. burgdorferi (9.7%), L. infantum (8.4%), C. burnetii (7.3%) and Trypanosoma evansi (6.6%) in dogs, and 242 (16.9%) ticks harbored Theileria lestoquardi (21.6%), Theileria ovis (20.0%) and Eh. ruminantium (0.3%) in sheep. Double, triple, and multiple co-infections represented 11% (90/816), 7.6% (62/816), and 10.3% (84/816), respectively in Rh. rutilus from dogs, whereas double and triple co-infections represented 30.2% (73/242) and 2.1% (5/242), respectively in Rh. rutilus from sheep. Approximately 92.5% (1,355/1,465) of Rhipicephalus annulatus ticks of cattle carried a burden of Anaplasma marginale (21.3%), Babesia bigemina (18.2%), Babesia bovis (14.0%), Borrelia theleri (12.8%), R. africae (12.4%), Th. annulata (8.7%), Bo. burgdorferi (2.7%), and Eh. ruminantium (2.5%). Double, triple, and multiple co-infections represented 1.8% (25/1,355), 11.5% (156/1,355), and 12.9% (175/1,355), respectively. The detected pathogens' sequences had 98.76-100% similarity to the available database with genetic divergence ranged between 0.0001 to 0.0009% to closest sequences from other African, Asian, and European countries. Phylogenetic analysis revealed close similarities between the detected pathogens and other isolates mostly from African and Asian countries.
Conclusions/significance: Continuous PCR-detection of pathogens transmitted by ticks is necessary to overcome the consequences of these infection to the hosts. More restrictions should be applied from the Egyptian authorities on animal importations to limit the emergence and re-emergence of tick-borne pathogens in the country. This is the first in-depth investigation of TBPs in Egypt.
Copyright: © 2024 Senbill et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures










Similar articles
-
Tick species identification and molecular detection of tick-borne pathogens in blood and ticks collected from cattle in Egypt.Ticks Tick Borne Dis. 2021 May;12(3):101676. doi: 10.1016/j.ttbdis.2021.101676. Epub 2021 Jan 26. Ticks Tick Borne Dis. 2021. PMID: 33540276
-
Tissue-specific localization of tick-borne pathogens in ticks collected from camels in Kenya: insights into vector competence.Front Cell Infect Microbiol. 2024 Apr 18;14:1382228. doi: 10.3389/fcimb.2024.1382228. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38698904 Free PMC article.
-
Molecular characterization of tick-borne bacterial and protozoan pathogens in parasitic ticks from Xinjiang, China.Parasit Vectors. 2025 Jun 4;18(1):207. doi: 10.1186/s13071-025-06857-1. Parasit Vectors. 2025. PMID: 40468420 Free PMC article.
-
Tick-borne pathogens in camels: A systematic review and meta-analysis of the prevalence in dromedaries.Ticks Tick Borne Dis. 2024 Jan;15(1):102268. doi: 10.1016/j.ttbdis.2023.102268. Epub 2023 Sep 26. Ticks Tick Borne Dis. 2024. PMID: 37769585
-
Hard ticks (Acari: Ixodidae) and tick-borne diseases of sheep and goats in Africa: A review.Ticks Tick Borne Dis. 2023 Nov;14(6):102232. doi: 10.1016/j.ttbdis.2023.102232. Epub 2023 Jul 31. Ticks Tick Borne Dis. 2023. PMID: 37531888 Review.
Cited by
-
Molecular prevalence of Borrelia burgdorferi, Ehrlichia canis, and Coxiella burnetii in dogs and associated ticks in Egypt: Emerging One Health challenging zoonoses.Vet World. 2024 Nov;17(11):2586-2594. doi: 10.14202/vetworld.2024.2586-2594. Epub 2024 Nov 22. Vet World. 2024. PMID: 39829650 Free PMC article.
-
Effects of copper/graphene oxide core-shell nanoparticles on Rhipicephalus ticks and their detoxification enzymes.Sci Rep. 2025 Jan 27;15(1):3334. doi: 10.1038/s41598-025-86560-4. Sci Rep. 2025. PMID: 39870717 Free PMC article.
References
-
- Davis GE, Hoogstraal H. The relapsing fevers: a survey of the tick-borne spirochetes of Egypt. J Egypt Public Health Assoc. 1954; 29: 139–143.
-
- Davis GE, Hoogstraal H. Etude sur la biologie du Spirochète Borrelia persica, trouvé chez la tique Ornithodorus tholozani (Argasinæ) récoltée dans le « Governorate » du désert occidental égyptien. Ann Parasitol Hum Comp. 1956; 31: 147–154. [In French]. doi: 10.1051/PARASITE/1956311147 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

