Protective function and differentiation cues of brain-resident CD8+ T cells during surveillance of latent Toxoplasma gondii infection
- PMID: 38838017
- PMCID: PMC11181119
- DOI: 10.1073/pnas.2403054121
Protective function and differentiation cues of brain-resident CD8+ T cells during surveillance of latent Toxoplasma gondii infection
Abstract
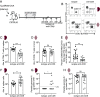
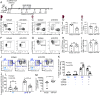
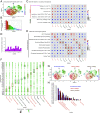
Chronic Toxoplasma gondii infection induces brain-resident CD8+ T cells (bTr), but the protective functions and differentiation cues of these cells remain undefined. Here, we used a mouse model of latent infection by T. gondii leading to effective CD8+ T cell-mediated parasite control. Thanks to antibody depletion approaches, we found that peripheral circulating CD8+ T cells are dispensable for brain parasite control during chronic stage, indicating that CD8+ bTr are able to prevent brain parasite reactivation. We observed that the retention markers CD69, CD49a, and CD103 are sequentially acquired by brain parasite-specific CD8+ T cells throughout infection and that a majority of CD69/CD49a/CD103 triple-positive (TP) CD8+ T cells also express Hobit, a transcription factor associated with tissue residency. This TP subset develops in a CD4+ T cell-dependent manner and is associated with effective parasite control during chronic stage. Conditional invalidation of Transporter associated with Antigen Processing (TAP)-mediated major histocompatibility complex (MHC) class I presentation showed that presentation of parasite antigens by glutamatergic neurons and microglia regulates the differentiation of CD8+ bTr into TP cells. Single-cell transcriptomic analyses revealed that resistance to encephalitis is associated with the expansion of stem-like subsets of CD8+ bTr. In summary, parasite-specific brain-resident CD8+ T cells are a functionally heterogeneous compartment which autonomously ensure parasite control during T. gondii latent infection and which differentiation is shaped by neuronal and microglial MHC I presentation. A more detailed understanding of local T cell-mediated immune surveillance of this common parasite is needed for harnessing brain-resident CD8+ T cells in order to enhance control of chronic brain infections.
Keywords: Toxoplasma gondii; apicomplexan parasite; brain; chronic infection; tissue-resident T cells.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Belz G. T., Kallies A., Effector and memory CD8+ T cell differentiation: Toward a molecular understanding of fate determination. Curr. Opin. Immunol. 22, 279–285 (2010). - PubMed
-
- Gebhardt T., et al. , Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009). - PubMed
-
- van Gisbergen K., Zens K. D., Munz C., T-cell memory in tissues. Eur. J. Immunol. 51, 1310–1324 (2021). - PubMed
-
- Day C. L., et al. , PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

