Paracoccus kondratievae produces poly(3-hydroxybutyrate) under elevated temperature conditions
- PMID: 38838099
- PMCID: PMC11150862
- DOI: 10.1111/1758-2229.13260
Paracoccus kondratievae produces poly(3-hydroxybutyrate) under elevated temperature conditions
Abstract
As part of ongoing efforts to discover novel polyhydroxyalkanoate-producing bacterial species, we embarked on characterizing the thermotolerant species, Paracoccus kondratievae, for biopolymer synthesis. Using traditional chemical and thermal characterization techniques, we found that P. kondratievae accumulates poly(3-hydroxybutyrate) (PHB), reaching up to 46.8% of the cell's dry weight after a 24-h incubation at 42°C. Although P. kondratievae is phylogenetically related to the prototypical polyhydroxyalkanoate producer, Paracoccus denitrificans, we observed significant differences in the PHB production dynamics between these two Paracoccus species. Notably, P. kondratievae can grow and produce PHB at elevated temperatures ranging from 42 to 47°C. Furthermore, P. kondratievae reaches its peak PHB content during the early stationary growth phase, specifically after 24 h of growth in a flask culture. This is then followed by a decline in the later stages of the stationary growth phase. The depolymerization observed in this growth phase is facilitated by the abundant presence of the PhaZ depolymerase enzyme associated with PHB granules. We observed the highest PHB levels when the cells were cultivated in a medium with glycerol as the sole carbon source and a carbon-to-nitrogen ratio of 10. Finally, we found that PHB production is induced as an osmotic stress response, similar to other polyhydroxyalkanoate-producing species.
© 2024 The Authors. Environmental Microbiology Reports published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors decalre no conflicts of interest.
Figures
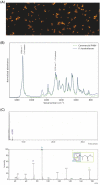



References
-
- An, J. , Ha, B. & Lee, S.K. (2023) Production of polyhydroxyalkanoates by the thermophile Cupriavidus cauae PHS1. Bioresource Technology, 371, 128627. - PubMed
-
- Baes, R. , Lemmens, L. , Mignon, K. , Carlier, M. & Peeters, E. (2020) Defining heat shock response for the thermoacidophilic model crenarchaeon Sulfolobus acidocaldarius . Extremophiles, 24, 681–692. - PubMed
-
- Chavan, S. , Yadav, B. , Tyagi, R.D. & Drogui, P. (2021) A review on production of polyhydroxyalkanoate (PHA) biopolyesters by thermophilic microbes using waste feedstocks. Bioresource Technology, 341, 125900. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

