Monodisperse nanosheet mesophases
- PMID: 38838140
- PMCID: PMC11152118
- DOI: 10.1126/sciadv.adk6452
Monodisperse nanosheet mesophases
Abstract
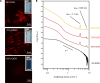
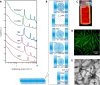
Self-assemblies of anisotropic colloidal particles into colloidal liquid crystals and well-defined superlattices are of great interest for hierarchical nanofabrications that are applicable for various functional materials. Inorganic nanosheets obtained by exfoliation of layered crystals have been highlighted as the intriguing colloidal units; however, the size polydispersity of the nanosheets has been preventing precise design of the assembled structures and their functions. Here, we demonstrate that the anionic titanate nanosheets with monodisperse size reversibly form very unusual superstructured mesophases through finely tunable weak attractive interactions between the nanosheets. Transmission electron microscopy, polarizing optical microscopy, small-angle x-ray scattering, and confocal laser scanning microscopy clarified the reversible formation of the mesophases (columnar nanofibers, columnar nematic liquid crystals, and columnar nanofiber bundles) as controlled by counter cations, nanosheet concentration, solvent, and temperature.
Figures


References
-
- Langmuir I., The role of attractive and repulsive forces in the formation of tactoids, thixotropic gels, protein crystals and coacervates. J. Chem. Phys. 6, 873–896 (1938).
-
- van der Kooij F. M., Kassapidou K., Lekkerkerker H. N. W., Liquid crystal phase transitions in suspensions of polydisperse plate-like particles. Nature 406, 868–871 (2000). - PubMed
-
- Gabriel J. C. P., Camerel F., Lemaire B. J., Desvaux H., Davidson P., Batail P., Swollen liquid-crystalline lamellar phase based on extended solid-like sheets. Nature 413, 504–508 (2001). - PubMed
-
- Mourad M. C. D., Wijnhoven J. E. G. J., Zand D. D., van der Beek D., Lekkerkerker H. N. W., Gelation versus liquid crystal phase transitions in suspensions of plate-like particles. Phil. Trans. R. Soc. A 364, 2807–2816 (2006). - PubMed
-
- Miyamoto N., Nakato T., Liquid crystalline nature of K4Nb6O17 nanosheet sols and their macroscopic alignment. Adv. Mater. 14, 1267–1270 (2002).
LinkOut - more resources
Full Text Sources

