Efficacy, reactogenicity, and safety of the adjuvanted recombinant zoster vaccine for the prevention of herpes zoster in Chinese adults ≥ 50 years: A randomized, placebo-controlled trial
- PMID: 38838170
- PMCID: PMC11253707
- DOI: 10.1080/21645515.2024.2351584
Efficacy, reactogenicity, and safety of the adjuvanted recombinant zoster vaccine for the prevention of herpes zoster in Chinese adults ≥ 50 years: A randomized, placebo-controlled trial
Abstract
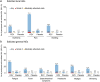
Phase III multi-country studies (ZOE-50/70) demonstrated that the adjuvanted recombinant zoster vaccine (RZV) was well tolerated and prevented herpes zoster (HZ) in healthy ≥ 50-year-olds, with a vaccine efficacy (VE) > 90% across age groups. These pivotal trials did not enroll participants from mainland China where RZV is licensed, therefore similar clinical data are missing for this population. In this phase IV observer-blind study (NCT04869982) conducted between 2021 and 2023 in China, immunocompetent and medically stable ≥ 50-year-olds were randomized 1:1 to receive two RZV or placebo doses, 2 months apart. This study assessed the VE (overall, as confirmatory objective, and descriptively by age category [50-69-year-olds/≥ 70-year-olds]), reactogenicity, and safety of RZV in this Chinese population. Of the 6138 enrolled participants, 99.2% completed the study. During a mean follow-up period of 15.2 (±1.1) months, 31 HZ episodes were confirmed (RZV = 0; placebo = 31) for an incidence rate of 0.0 vs 8.2 per 1000 person-years and an overall VE of 100% (89.82-100). The descriptive VE was 100% (85.29-100) for 50-69-year-olds and 100% (60.90-100) for ≥ 70-year-olds. Solicited adverse events (AEs) were more frequent in the RZV vs the placebo group (median duration: 1-3 days for both groups). Pain and fatigue were the most frequent local and general AEs (RZV: 72.1% and 43.4%; placebo: 9.2% and 5.3%). The frequencies of unsolicited AEs, serious AEs, potential immune-mediated diseases, and deaths were similar between both groups. RZV is well tolerated and efficacious in preventing HZ in Chinese ≥ 50-year-olds, consistent with efficacy studies including worldwide populations with similar age and medical characteristics.
Keywords: Herpes zoster; adjuvanted recombinant zoster vaccine; reactogenicity; safety; vaccine efficacy.
Plain language summary
What is the context? Herpes zoster, commonly known as shingles, is a painful rash resulting from the reactivation of the dormant virus causing chickenpox.Vaccines preventing shingles, such as Shingrix, were shown to be well tolerated and efficacious in healthy adults over 50 years of age from Europe, North and Latin America, Australia, and Asia (Taiwan, Hong Kong, Korea, Japan).However, data on real-world protective effect of Shingrix are limited in some regions where the vaccine is licensed for use, such as mainland China.What is new? We analyzed data from Chinese adults aged 50 years or older to determine the efficacy and safety of Shingrix.Around 6000 participants were divided in two equal groups to receive two doses of Shingrix or two doses of a placebo, given 2 months apart.We found that, during the study period, the vaccine was 100% efficacious in preventing shingles.We showed that the vaccine had an acceptable safety profile in this Chinese population.What is the impact? Shingrix is efficacious and well tolerated in Chinese adults over 50 years of age, as it is in similarly aged populations from other evaluated regions.
Conflict of interest statement
J.Z., J.S., N.P., and S.O.A. are/were employees of GSK at the time the study was designed, initiated, conducted, and/or completed. J.Z., J.S., N.P. and S.O.A. received stock options from GSK as part of their employee remuneration. D.A.E.P. was contracted by GSK from Aixial-Alten company to serve as a clinical lead for the study. X.S. and F.Z. have nothing to declare. All authors have no other financial and non-financial interests to declare.
Figures
References
-
- Centers for Disease Control and Prevention . Shingles (herpes zoster): clinical overview. 2023. [accessed 2023 Aug 18]. https://www.cdc.gov/shingles/hcp/clinical-overview.html.
-
- Lecrenier N, Beukelaers P, Colindres R, Curran D, De Kesel C, De Saegher JP, Didierlaurent AM, Ledent EY, Mols JF, Mrkvan T, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vaccines. 2018;17(7):619–10. doi:10.1080/14760584.2018.1495565. - DOI - PubMed
-
- Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapeliere P, Vassilev V, Ledent E, Heineman TC.. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein E subunit vaccine candidate in young and older adults. J Infect Dis. 2012;206(8):1280–90. doi:10.1093/infdis/jis497. - DOI - PubMed
-
- Chlibek R, Bayas JM, Collins H, de la Pinta ML, Ledent E, Mols JF, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥ 50 years of age. J Infect Dis. 2013;208(12):1953–61. doi:10.1093/infdis/jit365. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical