An open-label study of pemigatinib in cholangiocarcinoma: final results from FIGHT-202
- PMID: 38838500
- PMCID: PMC11190465
- DOI: 10.1016/j.esmoop.2024.103488
An open-label study of pemigatinib in cholangiocarcinoma: final results from FIGHT-202
Abstract
Background: Fibroblast growth factor receptor 2 (FGFR2) fusions and rearrangements are clinically actionable genomic alterations in cholangiocarcinoma (CCA). Pemigatinib is a selective, potent, oral inhibitor of FGFR1-3 and demonstrated efficacy in patients with previously treated, advanced/metastatic CCA with FGFR2 alterations in FIGHT-202 (NCT02924376). We report final outcomes from the extended follow-up period.
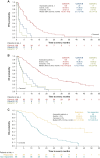
Patients and methods: The multicenter, open-label, single-arm, phase II FIGHT-202 study enrolled patients ≥18 years old with previously treated advanced/metastatic CCA with FGFR2 fusions or rearrangements (cohort A), other FGF/FGFR alterations (cohort B), or no FGF/FGFR alterations (cohort C). Patients received once-daily oral pemigatinib 13.5 mg in 21-day cycles (2 weeks on, 1 week off) until disease progression or unacceptable toxicity. The primary endpoint was objective response rate (ORR) in cohort A assessed as per RECIST v1.1 by an independent review committee; secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
Results: FIGHT-202 enrolled 147 patients (cohort A, 108; cohort B, 20; cohort C, 17; unconfirmed FGF/FGFR alterations, 2). By final analysis, 145 (98.6%) had discontinued treatment due to progressive disease (71.4%), withdrawal by patient (8.2%), or adverse events (AEs; 6.8%). Median follow-up was 45.4 months. The ORR in cohort A was 37.0% (95% confidence interval 27.9% to 46.9%); complete and partial responses were observed in 3 and 37 patients, respectively. Median DOR was 9.1 (6.0-14.5) months; median PFS and OS were 7.0 (6.1-10.5) months and 17.5 (14.4-22.9) months, respectively. The most common treatment-emergent AEs (TEAEs) were hyperphosphatemia (58.5%), alopecia (49.7%), and diarrhea (47.6%). Overall, 15 (10.2%) patients experienced TEAEs leading to pemigatinib discontinuation; intestinal obstruction and acute kidney injury (n = 2 each) occurred most frequently.
Conclusions: Pemigatinib demonstrated durable response and prolonged OS with manageable AEs in patients with previously treated, advanced/metastatic CCA with FGFR2 alterations in the extended follow-up period of FIGHT-202.
Keywords: fibroblast growth factor receptor; intrahepatic cholangiocarcinoma; next-generation sequencing; pemigatinib; precision medicine; targeted therapy.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Izquierdo-Sanchez L., Lamarca A., La Casta A., et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA registry. J Hepatol. 2022;76(5):1109–1121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

