A phase II study (AARDVARC) of AZD4635 in combination with durvalumab and cabazitaxel in patients with progressive, metastatic, castration-resistant prostate cancer
- PMID: 38838502
- PMCID: PMC11190476
- DOI: 10.1016/j.esmoop.2024.103446
A phase II study (AARDVARC) of AZD4635 in combination with durvalumab and cabazitaxel in patients with progressive, metastatic, castration-resistant prostate cancer
Abstract
Background: This phase II nonrandomized study evaluated the efficacy and safety of AZD4635 in combination with durvalumab (Arm A) or durvalumab plus cabazitaxel (Arm B) in patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel and ≥1 novel hormonal agent.
Patients and methods: The primary endpoint was radiographic progression-free survival (rPFS) per RECIST v1.1 (soft tissue) or the Prostate Cancer Clinical Trials Working Group 3 (bone). Secondary endpoints included safety, tolerability, overall survival, confirmed prostate-specific antigen (PSA50) response, pharmacokinetics, and objective response rate. Enrollment in Arm A was stopped following a sponsor decision unrelated to safety. The study was stopped based on the planned futility analysis due to low PSA50 response in Arm B.
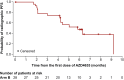
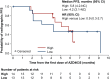
Results: In the final analysis (1 November 2021), 30 patients were treated (Arm A, n = 2; Arm B, n = 28). The median rPFS in Arm B was 5.8 months (95% confidence interval 4.2-not calculable). Median rPFS was 5.8 months versus 4.2 months for patients with high versus low blood-based adenosine signature. The most common treatment-related adverse events in Arm B were nausea (50.0%), diarrhea (46.4%), anemia and neutropenia (both 35.7%), asthenia (32.1%), and vomiting (28.6%). Overall, AZD4635 in combination with durvalumab or AZD4635 in combination with cabazitaxel and durvalumab showed limited efficacy in patients with mCRPC.
Conclusions: Although the safety profile of both combinations was consistent with known safety data of the individual agents, the results of this trial do not support further development of the combinations.
Keywords: AZD4635; cabazitaxel; durvalumab; metastatic castration-resistant prostate cancer; pharmacokinetics; safety.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure TAG: research funding, honoraria, and nonfinancial or other support from IPSEN, Adacap, Pfizer, Sanofi, EISAI, Lilly, Bayer, Janssen, BMS, Astellas, Novartis, Roche. MG: honoraria from Sanofi/Aventis and Alexion Pharmaceuticals for consulting or advisory role; travel, accommodations, expenses provided by AstraZeneca and Genentech; research funding paid to institution from Janssen, AstraZeneca, Genentech. CV: research funding paid to institution from Merck MSD; consulting fees from GSK, Astellas Pharma, Merck MSD, BMS, Leo-Pharma, Janssen, Cilag, Bayer, and AstraZeneca. GR: research funding paid to institution from Bayer; consulting fees from AAA, Astellas, Bayer, Sanofi, Janssen, AstraZeneca, and Pfizer. JZ: honoraria from AstraZeneca for advisory board and speaker bureau; advisory board for Bayer, Pfizer, and Dendreon; speaker bureau for Sanofi. MP: research funding paid to institution from Karyopharm; consulting fees from AstraZeneca, Exelixis, Oncocyte, Signatera, and Janssen. JMP: grants paid to institution from MSD, BMS, Janssen, Merk Serono, BeiGene; consulting fees from Novartis, Sanofi, Janssen, Astellas, BMS, MSD, and Roche. AA: employee of AstraZeneca and may own stock or stock options. GDJ: contractor for AstraZeneca. RC: employee of AstraZeneca and may own stock or stock options. ETG: employee of AstraZeneca and may own stock or stock options. JT: employee of AstraZeneca and may own stock or stock options. GP: employee of AstraZeneca and may own stock or stock options. RK: employee of AstraZeneca and may own stock or stock options. CS: research funding paid to institution by: Janssen, Astellas, Sanofi, Bayer, Sotio, and Dendreon; patents, consulting, or advisory role: Sanofi, Janssen, Astellas, Bayer, Genentech, Pfizer, Lilly; royalties and other intellectual property: Parthenolide (Indiana University); dimethylamino parthenolide (Leuchemix); Exelixis: abiraterone plus cabozantinib combination; FRAS1 SNP and tristetraprolin as biomarkers of lethal prostate cancer; stock or other ownership: Leuchemix.
Figures

References
-
- Bray F, Laversanne M, Sung H, et al. CA Cancer J Clin. 2024:1-35.
-
- Sayegh N., Swami U., Agarwal N. Recent advances in the management of metastatic prostate cancer. JCO Oncol Pract. 2022;18:45–55. - PubMed
-
- Tannock I.F., de Wit R., Berry W.R., et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

