Imaging brain glucose metabolism in vivo reveals propionate as a major anaplerotic substrate in pyruvate dehydrogenase deficiency
- PMID: 38838644
- PMCID: PMC11187753
- DOI: 10.1016/j.cmet.2024.05.002
Imaging brain glucose metabolism in vivo reveals propionate as a major anaplerotic substrate in pyruvate dehydrogenase deficiency
Abstract
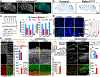
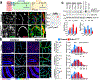
A vexing problem in mitochondrial medicine is our limited capacity to evaluate the extent of brain disease in vivo. This limitation has hindered our understanding of the mechanisms that underlie the imaging phenotype in the brain of patients with mitochondrial diseases and our capacity to identify new biomarkers and therapeutic targets. Using comprehensive imaging, we analyzed the metabolic network that drives the brain structural and metabolic features of a mouse model of pyruvate dehydrogenase deficiency (PDHD). As the disease progressed in this animal, in vivo brain glucose uptake and glycolysis increased. Propionate served as a major anaplerotic substrate, predominantly metabolized by glial cells. A combination of propionate and a ketogenic diet extended lifespan, improved neuropathology, and ameliorated motor deficits in these animals. Together, intermediary metabolism is quite distinct in the PDHD brain-it plays a key role in the imaging phenotype, and it may uncover new treatments for this condition.
Keywords: brain; glucose; imaging; ketogenic diet; metabolism; propionate; pyruvate; pyruvate dehydrogenase deficiency.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests K.R.K. is a founder with equity interest of Atish Technologies, Inc. and a member of the scientific advisory boards of NVision Imaging Technologies, Imaginostics, and Mi2. K.R.K. holds patents related to imaging and modulation of cellular metabolism. I.M.-V., K.R.K., C.R.-N., M.G.R., and A.K. are in the process of a patent application for the use of propionate as a therapeutic agent for PDHD and other neurometabolic diseases.
Figures





References
-
- Pirot N, Crahes M, Adle-Biassette H, Soares A, Bucourt M, Boutron A, Carbillon L, Mignot C, Trestard L, Bekri S, and Laquerriere A (2016). Phenotypic and Neuropathological Characterization of Fetal Pyruvate Dehydrogenase Deficiency. J Neuropathol Exp Neurol 75, 227–238. 10.1093/jnen/nlv022. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

