To each its own: Mechanisms of cross-talk between GPI biosynthesis and cAMP-PKA signaling in Candida albicans versus Saccharomyces cerevisiae
- PMID: 38838772
- PMCID: PMC11294708
- DOI: 10.1016/j.jbc.2024.107444
To each its own: Mechanisms of cross-talk between GPI biosynthesis and cAMP-PKA signaling in Candida albicans versus Saccharomyces cerevisiae
Abstract
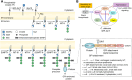
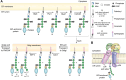
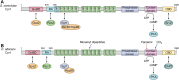
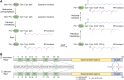
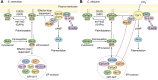
Candida albicans is an opportunistic fungal pathogen that can switch between yeast and hyphal morphologies depending on the environmental cues it receives. The switch to hyphal form is crucial for the establishment of invasive infections. The hyphal form is also characterized by the cell surface expression of hyphae-specific proteins, many of which are GPI-anchored and important determinants of its virulence. The coordination between hyphal morphogenesis and the expression of GPI-anchored proteins is made possible by an interesting cross-talk between GPI biosynthesis and the cAMP-PKA signaling cascade in the fungus; a parallel interaction is not found in its human host. On the other hand, in the nonpathogenic yeast, Saccharomyces cerevisiae, GPI biosynthesis is shut down when filamentation is activated and vice versa. This too is achieved by a cross-talk between GPI biosynthesis and cAMP-PKA signaling. How are diametrically opposite effects obtained from the cross-talk between two reasonably well-conserved pathways present ubiquitously across eukarya? This Review attempts to provide a model to explain these differences. In order to do so, it first provides an overview of the two pathways for the interested reader, highlighting the similarities and differences that are observed in C. albicans versus the well-studied S. cerevisiae model, before going on to explain how the different mechanisms of regulation are effected. While commonalities enable the development of generalized theories, it is hoped that a more nuanced approach, that takes into consideration species-specific differences, will enable organism-specific understanding of these processes and contribute to the development of targeted therapies.
Keywords: GPI-N-acetylglucosaminyltransferase; Ras; adenylyl cyclase; cAMP-PKA signaling; hyphae; pseudohyphae.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The author declares that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
The ER-Resident Ras Inhibitor 1 (Eri1) of Candida albicans Inhibits Hyphal Morphogenesis via the Ras-Independent cAMP-PKA Pathway.ACS Infect Dis. 2024 Oct 11;10(10):3528-3543. doi: 10.1021/acsinfecdis.4c00175. Epub 2024 Aug 9. ACS Infect Dis. 2024. PMID: 39119676
-
Ras signaling activates glycosylphosphatidylinositol (GPI) anchor biosynthesis via the GPI-N-acetylglucosaminyltransferase (GPI-GnT) in Candida albicans.J Biol Chem. 2018 Aug 3;293(31):12222-12238. doi: 10.1074/jbc.RA117.001225. Epub 2018 Jun 15. J Biol Chem. 2018. PMID: 29907567 Free PMC article.
-
Saccharomyces cerevisiae Ras2 restores filamentation but cannot activate the first step of GPI anchor biosynthesis in Candida albicans.Biochem Biophys Res Commun. 2019 Oct 1;517(4):755-761. doi: 10.1016/j.bbrc.2019.07.128. Epub 2019 Aug 8. Biochem Biophys Res Commun. 2019. PMID: 31402117
-
Generating anchors only to lose them: The unusual story of glycosylphosphatidylinositol anchor biosynthesis and remodeling in yeast and fungi.IUBMB Life. 2018 May;70(5):355-383. doi: 10.1002/iub.1734. IUBMB Life. 2018. PMID: 29679465 Review.
-
Messenger RNA transport in the opportunistic fungal pathogen Candida albicans.Curr Genet. 2017 Dec;63(6):989-995. doi: 10.1007/s00294-017-0707-6. Epub 2017 May 16. Curr Genet. 2017. PMID: 28512683 Free PMC article. Review.
References
-
- Komath S.S., Fujita M., Hart G.W., Ferguson M.A.J., Kinoshita T. In: Essentials of Glycobiology. 4th Ed. Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., et al., editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2022. Glycosylphosphatidylinositol anchors.
-
- Karim M., Singh G., Thakur S., Rana A., Rub A., Akhter Y. Evaluating complete surface-associated and secretory proteome of Leishmania donovani for discovering novel vaccines and diagnostic targets. Arch. Microbiol. 2022;204:604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

