Pharmacokinetics and Safety of Remdesivir in Pregnant and Nonpregnant Women With COVID-19: Results From IMPAACT 2032
- PMID: 38839047
- PMCID: PMC11481345
- DOI: 10.1093/infdis/jiae298
Pharmacokinetics and Safety of Remdesivir in Pregnant and Nonpregnant Women With COVID-19: Results From IMPAACT 2032
Abstract
Background: Pregnant people with coronavirus disease 2019 (COVID-19) experience higher risk for severe disease and adverse pregnancy outcomes, but no pharmacokinetic (PK) data exist to support dosing of COVID-19 therapeutics during pregnancy. We report PK and safety data for intravenous remdesivir in pregnancy.
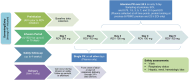
Methods: IMPAACT 2032 was a phase 4 prospective, open-label, nonrandomized opportunistic study of hospitalized pregnant and nonpregnant women receiving intravenous remdesivir as part of clinical care. Intensive PK sampling was performed on infusion days 3, 4, or 5 with collection of plasma and peripheral blood mononuclear cells (PBMCs). Safety data were recorded from first infusion through 4 weeks after last infusion and at delivery. Geometric mean ratios (GMR) (90% confidence intervals [CI]) of PK parameters between pregnant and nonpregnant women were calculated.
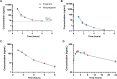
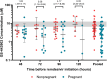
Results: Fifty-three participants initiated remdesivir (25 pregnant; median gestational age, 27.6 weeks; interquartile range, 24.9-31.0 weeks). Plasma exposures of remdesivir, its 2 major metabolites (GS-704277 and GS-441524), and the free remdesivir fraction were similar between pregnant and nonpregnant participants. Concentrations of the active triphosphate (GS-443902) in PBMCs increased 2.04-fold (90% CI, 1.35-3.03) with each additional infusion in nonpregnant versus pregnant participants. Three adverse events in nonpregnant participants were related to treatment (1 grade 3; 2 grade 2 resulting in treatment discontinuation). There were no treatment-related adverse pregnancy outcomes or congenital anomalies detected.
Conclusions: Plasma remdesivir PK parameters were comparable between pregnant and nonpregnant women, and no safety concerns were identified based on our limited data. These findings suggest no dose adjustments are indicated for intravenous remdesivir during pregnancy.
Clinical trials registration: NCT04582266.
Keywords: SARS-CoV-2; antiviral; pharmacology; pregnancy; remdesivir.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. K. M. B. has received consulting fees from ViiV Healthcare. K. B. has received research support from Gilead Sciences, Inc and ViiV Healthcare paid to her institution. D. S. has received research support from Gilead Sciences, Inc, Merck, and ViiV Healthcare paid to his institution. A. A. serves on scientific advisory boards for Gilead Sciences, Inc and ViiV Healthcare; is a site principal investigator for a multisite and investigator-initiated studies with Gilead Sciences, Inc; and is a consultant for Merck. E. V. C. is serving on a data and safety monitoring board for Melinta Pharmaceuticals. D. E. Y. was previously an unpaid technical advisor for the nonprofit Cover the Globe and Maipelo Trust. M. M. has received research support from Gilead Sciences, Inc, Merck, and ViiV Healthcare paid to his institution. R. H. and K. K. are employees of Gilead Sciences, Inc and hold stock in the company. J. D. M. has received research support from Gilead Sciences, Inc paid to his institution. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Dagan N, Barda N, Biron-Shental T, et al. . Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021; 27:1693–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

