Off-targets of BRAF inhibitors disrupt endothelial signaling and vascular barrier function
- PMID: 38839106
- PMCID: PMC11153892
- DOI: 10.26508/lsa.202402671
Off-targets of BRAF inhibitors disrupt endothelial signaling and vascular barrier function
Abstract
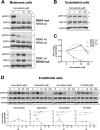
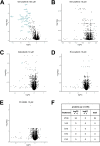
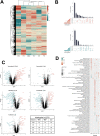
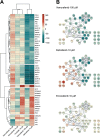
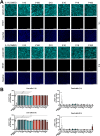
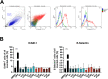
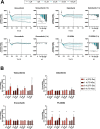
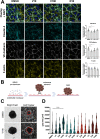
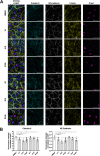
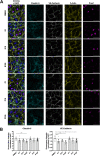
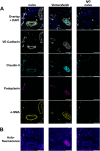
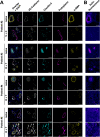
Targeted therapies against mutant BRAF are effectively used in combination with MEK inhibitors (MEKi) to treat advanced melanoma. However, treatment success is affected by resistance and adverse events (AEs). Approved BRAF inhibitors (BRAFi) show high levels of target promiscuity, which can contribute to these effects. The blood vessel lining is in direct contact with high plasma concentrations of BRAFi, but effects of the inhibitors in this cell type are unknown. Hence, we aimed to characterize responses to approved BRAFi for melanoma in the vascular endothelium. We showed that clinically approved BRAFi induced a paradoxical activation of endothelial MAPK signaling. Moreover, phosphoproteomics revealed distinct sets of off-targets per inhibitor. Endothelial barrier function and junction integrity were impaired upon treatment with vemurafenib and the next-generation dimerization inhibitor PLX8394, but not with dabrafenib or encorafenib. Together, these findings provide insights into the surprisingly distinct side effects of BRAFi on endothelial signaling and functionality. Better understanding of off-target effects could help to identify molecular mechanisms behind AEs and guide the continued development of therapies for BRAF-mutant melanoma.
© 2024 Bromberger et al.
Conflict of interest statement
JM Ressler received speaker honoraria from Bristol-Myers Squibb, Roche, Amgen and Novartis and travel support by Sanofi, Roche, and Bristol-Myers Squibb through institution. All other authors declare that they have no conflict of interest.
Figures


Similar articles
-
Inhibition of mutant BRAF splice variant signaling by next-generation, selective RAF inhibitors.Pigment Cell Melanoma Res. 2014 May;27(3):479-84. doi: 10.1111/pcmr.12218. Epub 2014 Feb 10. Pigment Cell Melanoma Res. 2014. PMID: 24422853 Free PMC article.
-
Targeting the PI3K/AKT/mTOR pathway overcomes the stimulating effect of dabrafenib on the invasive behavior of melanoma cells with acquired resistance to the BRAF inhibitor.Int J Oncol. 2016 Sep;49(3):1164-74. doi: 10.3892/ijo.2016.3594. Epub 2016 Jun 30. Int J Oncol. 2016. PMID: 27572607
-
Mutation-oriented profiling of autoinhibitory kinase conformations predicts RAF inhibitor efficacies.Proc Natl Acad Sci U S A. 2020 Dec 8;117(49):31105-31113. doi: 10.1073/pnas.2012150117. Epub 2020 Nov 23. Proc Natl Acad Sci U S A. 2020. PMID: 33229534 Free PMC article.
-
A Review of the Molecular Pathways Involved in Resistance to BRAF Inhibitors in Patients with Advanced-Stage Melanoma.Med Sci Monit. 2020 Apr 10;26:e920957. doi: 10.12659/MSM.920957. Med Sci Monit. 2020. PMID: 32273491 Free PMC article. Review.
-
Immunomodulatory effects of BRAF and MEK inhibitors: Implications for Melanoma therapy.Pharmacol Res. 2018 Oct;136:151-159. doi: 10.1016/j.phrs.2018.08.019. Epub 2018 Aug 23. Pharmacol Res. 2018. PMID: 30145328 Review.
Cited by
-
BRAF Modulates the Interplay Between Cell-Cell and Cell-Extracellular Matrix Adhesions in PECAM-1-Mediated Mechanotransduction.Int J Mol Sci. 2024 Oct 18;25(20):11234. doi: 10.3390/ijms252011234. Int J Mol Sci. 2024. PMID: 39457016 Free PMC article.
-
Increased Mitochondrial Superoxide Level Is Partially Associated With Vemurafenib-Induced Renal Tubular Toxicity.Basic Clin Pharmacol Toxicol. 2025 Apr;136(4):e70015. doi: 10.1111/bcpt.70015. Basic Clin Pharmacol Toxicol. 2025. PMID: 40018909 Free PMC article.
-
Drug repurposing candidates for amyotrophic lateral sclerosis using common and rare genetic variants.Brain Commun. 2025 May 9;7(3):fcaf184. doi: 10.1093/braincomms/fcaf184. eCollection 2025. Brain Commun. 2025. PMID: 40395632 Free PMC article.
References
-
- Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, et al. (2016) Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 17: 1248–1260. 10.1016/S1470-2045(16)30122-X - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
