Targeting the oncogenic m6A demethylase FTO suppresses tumourigenesis and potentiates immune response in hepatocellular carcinoma
- PMID: 38839271
- PMCID: PMC11672076
- DOI: 10.1136/gutjnl-2024-331903
Targeting the oncogenic m6A demethylase FTO suppresses tumourigenesis and potentiates immune response in hepatocellular carcinoma
Abstract
Objective: Fat mass and obesity-associated protein (FTO), an eraser of N 6-methyadenosine (m6A), plays oncogenic roles in various cancers. However, its role in hepatocellular carcinoma (HCC) is unclear. Furthermore, small extracellular vesicles (sEVs, or exosomes) are critical mediators of tumourigenesis and metastasis, but the relationship between FTO-mediated m6A modification and sEVs in HCC is unknown.
Design: The functions and mechanisms of FTO and glycoprotein non-metastatic melanoma protein B (GPNMB) in HCC progression were investigated in vitro and in vivo. Neutralising antibody of syndecan-4 (SDC4) was used to assess the significance of sEV-GPNMB. FTO inhibitor CS2 was used to examine the effects on anti-PD-1 and sorafenib treatment.
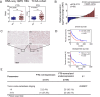
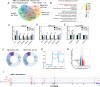
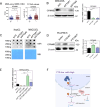
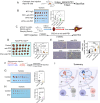
Results: FTO expression was upregulated in patient HCC tumours. Functionally, FTO promoted HCC cell proliferation, migration and invasion in vitro, and tumour growth and metastasis in vivo. FTO knockdown enhanced the activation and recruitment of tumour-infiltrating CD8+ T cells. Furthermore, we identified GPNMB to be a downstream target of FTO, which reduced the m6A abundance of GPNMB, hence, stabilising it from degradation by YTH N 6-methyladenosine RNA binding protein F2. Of note, GPNMB was packaged into sEVs derived from HCC cells and bound to the surface receptor SDC4 of CD8+ T cells, resulting in the inhibition of CD8+ T cell activation. A potential FTO inhibitor, CS2, suppresses the oncogenic functions of HCC cells and enhances the sensitivity of anti-PD-1 and sorafenib treatment.
Conclusion: Targeting the FTO/m6A/GPNMB axis could significantly suppress tumour growth and metastasis, and enhance immune activation, highlighting the potential of targeting FTO signalling with effective inhibitors for HCC therapy.
Keywords: CELL BIOLOGY; GENE REGULATION; HEPATOCELLULAR CARCINOMA; IMMUNE RESPONSE.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
