Protective function of sclerosing cholangitis on IBD
- PMID: 38839272
- PMCID: PMC11287650
- DOI: 10.1136/gutjnl-2023-330856
Protective function of sclerosing cholangitis on IBD
Abstract
Objective: There is a strong clinical association between IBD and primary sclerosing cholangitis (PSC), a chronic disease of the liver characterised by biliary inflammation that leads to strictures and fibrosis. Approximately 60%-80% of people with PSC will also develop IBD (PSC-IBD). One hypothesis explaining this association would be that PSC drives IBD. Therefore, our aim was to test this hypothesis and to decipher the underlying mechanism.
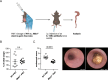
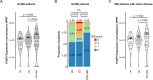
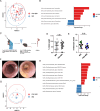
Design: Colitis severity was analysed in experimental mouse models of colitis and sclerosing cholangitis, and people with IBD and PSC-IBD. Foxp3+ Treg-cell infiltration was assessed by qPCR and flow cytometry. Microbiota profiling was carried out from faecal samples of people with IBD, PSC-IBD and mouse models recapitulating these diseases. Faecal microbiota samples collected from people with IBD and PSC-IBD were transplanted into germ-free mice followed by colitis induction.
Results: We show that sclerosing cholangitis attenuated IBD in mouse models. Mechanistically, sclerosing cholangitis causes an altered intestinal microbiota composition, which promotes Foxp3+ Treg-cell expansion, and thereby protects against IBD. Accordingly, sclerosing cholangitis promotes IBD in the absence of Foxp3+ Treg cells. Furthermore, people with PSC-IBD have an increased Foxp3+ expression in the colon and an overall milder IBD severity. Finally, by transplanting faecal microbiota into gnotobiotic mice, we showed that the intestinal microbiota of people with PSC protects against colitis.
Conclusion: This study shows that PSC attenuates IBD and provides a comprehensive insight into the mechanisms involved in this effect.
Keywords: COLONIC MICROFLORA; Cholangitis; INFLAMMATORY BOWEL DISEASE; PRIMARY SCLEROSING CHOLANGITIS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
