Brain Network Interconnectivity Dynamics Explain Metacognitive Differences in Listening Behavior
- PMID: 38839303
- PMCID: PMC11293451
- DOI: 10.1523/JNEUROSCI.2322-23.2024
Brain Network Interconnectivity Dynamics Explain Metacognitive Differences in Listening Behavior
Erratum in
-
Erratum: Alavash and Obleser, "Brain Network Interconnectivity Dynamics Explain Metacognitive Differences in Listening Behavior".J Neurosci. 2025 May 28;45(22):e0893252025. doi: 10.1523/JNEUROSCI.0893-25.2025. J Neurosci. 2025. PMID: 40419329 Free PMC article. No abstract available.
Abstract
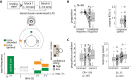
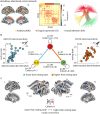
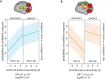
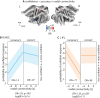
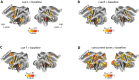
Complex auditory scenes pose a challenge to attentive listening, rendering listeners slower and more uncertain in their perceptual decisions. How can we explain such behaviors from the dynamics of cortical networks that pertain to the control of listening behavior? We here follow up on the hypothesis that human adaptive perception in challenging listening situations is supported by modular reconfiguration of auditory-control networks in a sample of N = 40 participants (13 males) who underwent resting-state and task functional magnetic resonance imaging (fMRI). Individual titration of a spatial selective auditory attention task maintained an average accuracy of ∼70% but yielded considerable interindividual differences in listeners' response speed and reported confidence in their own perceptual decisions. Whole-brain network modularity increased from rest to task by reconfiguring auditory, cinguloopercular, and dorsal attention networks. Specifically, interconnectivity between the auditory network and cinguloopercular network decreased during the task relative to the resting state. Additionally, interconnectivity between the dorsal attention network and cinguloopercular network increased. These interconnectivity dynamics were predictive of individual differences in response confidence, the degree of which was more pronounced after incorrect judgments. Our findings uncover the behavioral relevance of functional cross talk between auditory and attentional-control networks during metacognitive assessment of one's own perception in challenging listening situations and suggest two functionally dissociable cortical networked systems that shape the considerable metacognitive differences between individuals in adaptive listening behavior.
Keywords: adaptive listening behavior; cinguloopercular network; confidence; dorsal attention network; fMRI; metacognitive differences.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Modular reconfiguration of an auditory control brain network supports adaptive listening behavior.Proc Natl Acad Sci U S A. 2019 Jan 8;116(2):660-669. doi: 10.1073/pnas.1815321116. Epub 2018 Dec 26. Proc Natl Acad Sci U S A. 2019. PMID: 30587584 Free PMC article. Clinical Trial.
-
Dynamic large-scale connectivity of intrinsic cortical oscillations supports adaptive listening in challenging conditions.PLoS Biol. 2021 Oct 11;19(10):e3001410. doi: 10.1371/journal.pbio.3001410. eCollection 2021 Oct. PLoS Biol. 2021. PMID: 34634031 Free PMC article.
-
How restful is it with all that noise? Comparison of Interleaved silent steady state (ISSS) and conventional imaging in resting-state fMRI.Neuroimage. 2017 Feb 15;147:726-735. doi: 10.1016/j.neuroimage.2016.11.065. Epub 2016 Nov 27. Neuroimage. 2017. PMID: 27902936
-
The spatio-temporal dynamics of deviance and target detection in the passive and active auditory oddball paradigm: a sLORETA study.BMC Neurosci. 2018 Apr 19;19(1):25. doi: 10.1186/s12868-018-0422-3. BMC Neurosci. 2018. PMID: 29673322 Free PMC article.
-
Mapping cognitive and emotional networks in neurosurgical patients using resting-state functional magnetic resonance imaging.Neurosurg Focus. 2020 Feb 1;48(2):E9. doi: 10.3171/2019.11.FOCUS19773. Neurosurg Focus. 2020. PMID: 32006946 Free PMC article. Review.
Cited by
-
Neurophysiology of Effortful Listening: Decoupling Motivational Modulation from Task Demands.J Neurosci. 2024 Oct 30;44(44):e0589242024. doi: 10.1523/JNEUROSCI.0589-24.2024. J Neurosci. 2024. PMID: 39261007 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
