Broad specificity of monoclonal IgA (TEPC15-IgA) for enteric bacteria via phosphorylcholine-mediated interaction
- PMID: 38839348
- PMCID: PMC11251817
- DOI: 10.1292/jvms.23-0441
Broad specificity of monoclonal IgA (TEPC15-IgA) for enteric bacteria via phosphorylcholine-mediated interaction
Abstract
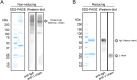
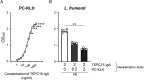
Immunoglobulin A (IgA) is notable for its broad specificity toward multiple bacteria. Phosphorylcholine (PC) plays a role in the infection of pathogenic bacteria carrying PC and in the induction of IgA responses in the host immune system. The commercially available mouse monoclonal IgA, TEPC15-IgA, is a distinctive antibody with specificity for PC, warranting further exploration of its response to PC-bearing enteric bacteria. In this study, using 17 different enteric bacteria, including 3 aerobic and 14 anerobic bacteria that could be cultured in vitro, we confirmed that TEPC15-IgA recognizes 4 bacterial species: Lactobacillus taiwanensis, Limosilactobacillus frumenti, Streptococcus infantis, and Escherichia coli, although reactivity varied. Interestingly, TEPC15-IgA did not react with four of six Lactobacillus species used. Moreover, distinct target molecules associated with PC in L. taiwanensis and L. frumenti were evident, differing in molecular weight. These findings suggest that the natural generation of PC-specific IgA could prevent PC-mediated infections and potentially facilitate the formation of a microflora rich in indigenous bacteria with PC, particularly in the gastrointestinal tract.
Keywords: enteric bacteria; immune system; immunoglobulin A; phosphorylcholine.
Conflict of interest statement
None of the authors of this paper has any financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Figures



Similar articles
-
Immune response to phosphorylcholine. IX. Characterization of hybridoma anti-TEPC15 antibodies.J Immunol. 1982 Feb;128(2):595-9. J Immunol. 1982. PMID: 7033381
-
Shared idiotypy between phosphorylcholine-specific antibody and acetylcholinesterase detectable by a monoclonal antibody.J Immunol. 1985 Feb;134(2):1053-8. J Immunol. 1985. PMID: 3871206
-
Binding of phosphorylcholine by non-immunoglobulin molecules on mouse B cells.J Immunol. 1983 Jul;131(1):365-9. J Immunol. 1983. PMID: 6863921
-
The mucosal immune system of the upper respiratory tract and recent progress in mucosal vaccines.Auris Nasus Larynx. 2022 Feb;49(1):1-10. doi: 10.1016/j.anl.2021.07.003. Epub 2021 Jul 23. Auris Nasus Larynx. 2022. PMID: 34304944 Review.
-
The IgE antibody response to the phosphorylcholine hapten.Int Rev Immunol. 1987 Jan;2(1):93-115. doi: 10.3109/08830188709044749. Int Rev Immunol. 1987. PMID: 3333780 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

