Effectiveness of COVID-19 vaccines administered in the 2023 autumnal campaigns in Europe: Results from the VEBIS primary care test-negative design study, September 2023-January 2024
- PMID: 38839521
- PMCID: PMC11252666
- DOI: 10.1016/j.vaccine.2024.05.067
Effectiveness of COVID-19 vaccines administered in the 2023 autumnal campaigns in Europe: Results from the VEBIS primary care test-negative design study, September 2023-January 2024
Erratum in
-
Corrigendum to "Effectiveness of COVID-19 vaccines administered in the 2023 autumnal campaigns in Europe: results from the VEBIS primary care test-negative design study, September 2023-January 2024" [Vaccine 42(19) (2024)].Vaccine. 2024 Oct 3;42(23):126089. doi: 10.1016/j.vaccine.2024.06.056. Epub 2024 Jul 8. Vaccine. 2024. PMID: 38971666 Free PMC article. No abstract available.
Abstract
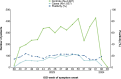
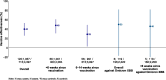
In autumn 2023, European vaccination campaigns predominantly administered XBB.1.5 vaccine. In a European multicentre study, we estimated 2023 COVID-19 vaccine effectiveness (VE) against laboratory-confirmed symptomatic infection at primary care level between September 2023 and January 2024. Using a test-negative case-control design, we estimated VE in the target group for COVID-19 vaccination overall and by time since vaccination. We included 1057 cases and 4397 controls. Vaccine effectiveness was 40 % (95 % CI: 26-53 %) overall, 48 % (95 % CI: 31-61 %) among those vaccinated < 6 weeks of onset and 29 % (95 % CI: 3-49 %) at 6-14 weeks. Our results suggest that COVID-19 vaccines administered to target groups during the autumn 2023 campaigns showed clinically significant effectiveness against laboratory-confirmed, medically attended symptomatic SARS-CoV-2 infection in the 3 months following vaccination. A longer study period will allow for further variant-specific COVID-19 VE estimates, better understanding decline in VE and informing booster administration policies.
Keywords: COVID-19; Europe; Multicentre study; SARS-CoV-2; Test-negative design; Vaccine effectiveness.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- European Medicines Agency. COVID-19 vaccines: authorised [Internet]. [cited 2024 Mar 21]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr....
-
- European Centre for Disease Prevention and Control. EMA and ECDC statement on updating COVID-19 vaccines to target new SARS-CoV-2 virus variants [Internet]. [cited 2024 Feb 12]. Available from: file:///C:/Users/esthe/Downloads/ecdc-ema_statement_on_updating_covid-19_vaccines_composition_for_new_sars-cov-2_virus_variants_en(1).pdf.
-
- European Centre for Disease Prevention and Control. Interim COVID-19 vaccination coverage in the EU/EEA during the 2023–24 season campaigns [Internet]. 2024 Jan [cited 2024 Apr 19]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/interim-vaccine....
-
- European Centre for Disease Prevention and Control. European Respiratory Virus Surveillance Summary (ERVISS), 2024, Week 07. [Internet]. 202Available from: https://erviss.org/.
-
- Valenciano M., Ciancio B. I-MOVE study team. I-MOVE: a European network to measure the effectiveness of influenza vaccines. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2012;17(39) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

