Neonatal and Maternal Ichthyosiform Dermopathy in Association with Kava Use during Pregnancy
- PMID: 38839731
- PMCID: PMC11288220
- DOI: 10.1007/s13181-024-01016-x
Neonatal and Maternal Ichthyosiform Dermopathy in Association with Kava Use during Pregnancy
Abstract
Introduction: Kava, a substance derived from the Piper methysticum plant, is enjoying a surge in popularity in the United States due to its purported anxiolytic and analgesic effects. Though ichthyosiform dermopathy is a known adverse effect associated with chronic kava exposure in adults, dermopathy in a newborn due to maternal kava use has not yet been described.
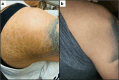
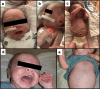
Case report: This is a case of a 41-year-old woman who was taking a combination kava/kratom product throughout her pregnancy. She developed an ichthyosiform dermopathy that resolved after she stopped using the product postpartum. Her male infant had a neonatal course complicated by both neonatal opioid withdrawal syndrome, attributed to maternal kratom and buprenorphine use, as well as a diffuse ichthyosiform rash similar to descriptions of kava ichthyosiform dermopathy in adults. His neonatal course was complicated by Group B streptococcus and Serratia marscecens bacteremia (treated with antibiotics) and seizures (treated with lorazepam and phenobarbital). His rash resolved completely by day of life 22. At 9-month outpatient follow-up, he had no dermatologic abnormalities or rash recurrence.
Discussion: Maternal kava use during pregnancy may cause fetal dermopathy presenting as an acquired ichthyosis. More public education is needed about the potential consequences of kava use, particularly during pregnancy.
Keywords: Dermopathy; Ichthyosis; Kava; Newborn; Pregnancy.
© 2024. American College of Medical Toxicology.
Conflict of interest statement
Hannah Spungen, Kartik Mody, Becky Micetic, Christine Wade, and A. Min Kang declare that they have no conflict of interest.
Figures

Similar articles
-
Severe kava withdrawal managed with phenobarbital.Am J Emerg Med. 2025 Jun 16:S0735-6757(25)00397-3. doi: 10.1016/j.ajem.2025.06.016. Online ahead of print. Am J Emerg Med. 2025. PMID: 40541460
-
Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes.Cochrane Database Syst Rev. 2018 Jul 24;7(7):CD010564. doi: 10.1002/14651858.CD010564.pub2. Cochrane Database Syst Rev. 2018. PMID: 30039871 Free PMC article.
-
Sedatives for opiate withdrawal in newborn infants.Cochrane Database Syst Rev. 2002;(3):CD002053. doi: 10.1002/14651858.CD002053. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2005 Jul 20;(3):CD002053. doi: 10.1002/14651858.CD002053.pub2. PMID: 12137641 Updated.
-
Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour.Cochrane Database Syst Rev. 2017 Feb 3;2(2):CD006066. doi: 10.1002/14651858.CD006066.pub3. Cochrane Database Syst Rev. 2017. PMID: 28157275 Free PMC article.
-
Iodine supplementation for women during the preconception, pregnancy and postpartum period.Cochrane Database Syst Rev. 2017 Mar 5;3(3):CD011761. doi: 10.1002/14651858.CD011761.pub2. Cochrane Database Syst Rev. 2017. PMID: 28260263 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

