Coexistence of state, choice, and sensory integration coding in barrel cortex LII/III
- PMID: 38839747
- PMCID: PMC11153558
- DOI: 10.1038/s41467-024-49129-9
Coexistence of state, choice, and sensory integration coding in barrel cortex LII/III
Abstract
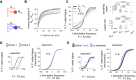
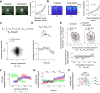
During perceptually guided decisions, correlates of choice are found as upstream as in the primary sensory areas. However, how well these choice signals align with early sensory representations, a prerequisite for their interpretation as feedforward substrates of perception, remains an open question. We designed a two alternative forced choice task (2AFC) in which male mice compared stimulation frequencies applied to two adjacent vibrissae. The optogenetic silencing of individual columns in the primary somatosensory cortex (wS1) resulted in predicted shifts of psychometric functions, demonstrating that perception depends on focal, early sensory representations. Functional imaging of layer II/III single neurons revealed mixed coding of stimuli, choices and engagement in the task. Neurons with multi-whisker suppression display improved sensory discrimination and had their activity increased during engagement in the task, enhancing selectively representation of the signals relevant to solving the task. From trial to trial, representation of stimuli and choice varied substantially, but mostly orthogonally to each other, suggesting that perceptual variability does not originate from wS1 fluctuations but rather from downstream areas. Together, our results highlight the role of primary sensory areas in forming a reliable sensory substrate that could be used for flexible downstream decision processes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
A latent pool of neurons silenced by sensory-evoked inhibition can be recruited to enhance perception.Neuron. 2024 Jul 17;112(14):2386-2403.e6. doi: 10.1016/j.neuron.2024.04.015. Epub 2024 May 9. Neuron. 2024. PMID: 38729150 Free PMC article.
-
Origins of choice-related activity in mouse somatosensory cortex.Nat Neurosci. 2016 Jan;19(1):127-34. doi: 10.1038/nn.4183. Epub 2015 Dec 7. Nat Neurosci. 2016. PMID: 26642088 Free PMC article.
-
A Non-canonical Feedback Circuit for Rapid Interactions between Somatosensory Cortices.Cell Rep. 2018 May 29;23(9):2718-2731.e6. doi: 10.1016/j.celrep.2018.04.115. Cell Rep. 2018. PMID: 29847801 Free PMC article.
-
Neuronal Circuits in Barrel Cortex for Whisker Sensory Perception.Physiol Rev. 2021 Jan 1;101(1):353-415. doi: 10.1152/physrev.00019.2019. Epub 2020 Aug 20. Physiol Rev. 2021. PMID: 32816652 Review.
-
Representation of tactile scenes in the rodent barrel cortex.Neuroscience. 2018 Jan 1;368:81-94. doi: 10.1016/j.neuroscience.2017.08.039. Epub 2017 Aug 23. Neuroscience. 2018. PMID: 28843997 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

