Skin-inspired, sensory robots for electronic implants
- PMID: 38839748
- PMCID: PMC11153219
- DOI: 10.1038/s41467-024-48903-z
Skin-inspired, sensory robots for electronic implants
Abstract
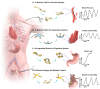
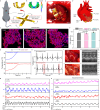
Drawing inspiration from cohesive integration of skeletal muscles and sensory skins in vertebrate animals, we present a design strategy of soft robots, primarily consisting of an electronic skin (e-skin) and an artificial muscle. These robots integrate multifunctional sensing and on-demand actuation into a biocompatible platform using an in-situ solution-based method. They feature biomimetic designs that enable adaptive motions and stress-free contact with tissues, supported by a battery-free wireless module for untethered operation. Demonstrations range from a robotic cuff for detecting blood pressure, to a robotic gripper for tracking bladder volume, an ingestible robot for pH sensing and on-site drug delivery, and a robotic patch for quantifying cardiac function and delivering electrotherapy, highlighting the application versatilities and potentials of the bio-inspired soft robots. Our designs establish a universal strategy with a broad range of sensing and responsive materials, to form integrated soft robots for medical technology and beyond.
© 2024. The Author(s).
Conflict of interest statement
The University of North Carolina at Chapel Hill (No. 035052/602726: filed on Oct. 13, 2023) filed a provisional patent application, titled “Skin-inspired, sensory robots for electronic implants”, surrounding this work, where W.B. and L.Z. are the co-inventors. The remaining authors declare no competing interests.
Figures




Update of
-
Skin-inspired, sensory robots for electronic implants.Res Sq [Preprint]. 2023 Dec 22:rs.3.rs-3665801. doi: 10.21203/rs.3.rs-3665801/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Jun 5;15(1):4777. doi: 10.1038/s41467-024-48903-z. PMID: 38196588 Free PMC article. Updated. Preprint.
References
-
- Choi H, et al. Adhesive bioelectronics for sutureless epicardial interfacing. Nat. Electron. 2023;6:779–789. doi: 10.1038/s41928-023-01023-w. - DOI
MeSH terms
Grants and funding
- 1R01EB034332-01/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- P30 CA016086/CA/NCI NIH HHS/United States
- ECCS-2025064/National Science Foundation (NSF)
- R01 EB034332/EB/NIBIB NIH HHS/United States
- ECCS-2139659/National Science Foundation (NSF)
LinkOut - more resources
Full Text Sources

