A candidate sex determination locus in amphibians which evolved by structural variation between X- and Y-chromosomes
- PMID: 38839766
- PMCID: PMC11153619
- DOI: 10.1038/s41467-024-49025-2
A candidate sex determination locus in amphibians which evolved by structural variation between X- and Y-chromosomes
Abstract
Most vertebrates develop distinct females and males, where sex is determined by repeatedly evolved environmental or genetic triggers. Undifferentiated sex chromosomes and large genomes have caused major knowledge gaps in amphibians. Only a single master sex-determining gene, the dmrt1-paralogue (dm-w) of female-heterogametic clawed frogs (Xenopus; ZW♀/ZZ♂), is known across >8740 species of amphibians. In this study, by combining chromosome-scale female and male genomes of a non-model amphibian, the European green toad, Bufo(tes) viridis, with ddRAD- and whole genome pool-sequencing, we reveal a candidate master locus, governing a male-heterogametic system (XX♀/XY♂). Targeted sequencing across multiple taxa uncovered structural X/Y-variation in the 5'-regulatory region of the gene bod1l, where a Y-specific non-coding RNA (ncRNA-Y), only expressed in males, suggests that this locus initiates sex-specific differentiation. Developmental transcriptomes and RNA in-situ hybridization show timely and spatially relevant sex-specific ncRNA-Y and bod1l-gene expression in primordial gonads. This coincided with differential H3K4me-methylation in pre-granulosa/pre-Sertoli cells, pointing to a specific mechanism of amphibian sex determination.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
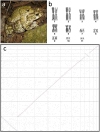



Similar articles
-
Mammalian sex--Origin and evolution of the Y chromosome and SRY.Semin Cell Dev Biol. 2007 Jun;18(3):389-400. doi: 10.1016/j.semcdb.2007.02.007. Epub 2007 Feb 24. Semin Cell Dev Biol. 2007. PMID: 17400006 Review.
-
Sex chromosome conservation, DMRT1 phylogeny and gonad morphology in diploid Palearctic green toads (Bufo viridis subgroup).Cytogenet Genome Res. 2014;144(4):315-24. doi: 10.1159/000380841. Epub 2015 Mar 24. Cytogenet Genome Res. 2014. PMID: 25823515
-
Dynamics of vertebrate sex chromosome evolution: from equal size to giants and dwarfs.Chromosoma. 2016 Jun;125(3):553-71. doi: 10.1007/s00412-015-0569-y. Epub 2015 Dec 29. Chromosoma. 2016. PMID: 26715206 Review.
-
Sex chromosomes, sex-linked genes, and sex determination in the vertebrate class amphibia.EXS. 2001;(91):143-76. doi: 10.1007/978-3-0348-7781-7_8. EXS. 2001. PMID: 11301597 Review.
-
Cytogenetic Insights into the Evolution of Chromosomes and Sex Determination Reveal Striking Homology of Turtle Sex Chromosomes to Amphibian Autosomes.Cytogenet Genome Res. 2016;148(4):292-304. doi: 10.1159/000447478. Epub 2016 Jul 16. Cytogenet Genome Res. 2016. PMID: 27423490
Cited by
-
Comparative molecular and conventional cytogenetic analyses of three species of Rhinella (Anura; Bufonidae).PLoS One. 2024 Aug 15;19(8):e0308785. doi: 10.1371/journal.pone.0308785. eCollection 2024. PLoS One. 2024. PMID: 39146271 Free PMC article.
-
A chromosome-level genome assembly of the European green toad (Bufotes viridis).G3 (Bethesda). 2025 Mar 18;15(3):jkaf002. doi: 10.1093/g3journal/jkaf002. G3 (Bethesda). 2025. PMID: 39969399 Free PMC article.
-
FISH mapping in Xenopus pygmaeus refines understanding of genomic rearrangements and reveals jumping NORs in African clawed frogs.Heredity (Edinb). 2025 Apr;134(3-4):209-220. doi: 10.1038/s41437-025-00749-x. Epub 2025 Mar 1. Heredity (Edinb). 2025. PMID: 40025138 Free PMC article.
-
The Amphibian Genomics Consortium: advancing genomic and genetic resources for amphibian research and conservation.bioRxiv [Preprint]. 2024 Oct 3:2024.06.27.601086. doi: 10.1101/2024.06.27.601086. bioRxiv. 2024. Update in: BMC Genomics. 2024 Nov 1;25(1):1025. doi: 10.1186/s12864-024-10899-7. PMID: 39005434 Free PMC article. Updated. Preprint.
-
The Amphibian Genomics Consortium: advancing genomic and genetic resources for amphibian research and conservation.BMC Genomics. 2024 Nov 1;25(1):1025. doi: 10.1186/s12864-024-10899-7. BMC Genomics. 2024. PMID: 39487448 Free PMC article. Review.
References
-
- Beukeboom, L. W. & Perrin, N. The evolution of sex determination (Oxford University Press, 2014).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

