Supramolecular assembly activated single-molecule phosphorescence resonance energy transfer for near-infrared targeted cell imaging
- PMID: 38839843
- PMCID: PMC11153566
- DOI: 10.1038/s41467-024-49238-5
Supramolecular assembly activated single-molecule phosphorescence resonance energy transfer for near-infrared targeted cell imaging
Abstract
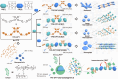
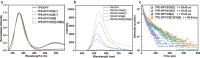
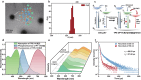
Pure organic phosphorescence resonance energy transfer is a research hotspot. Herein, a single-molecule phosphorescence resonance energy transfer system with a large Stokes shift of 367 nm and near-infrared emission is constructed by guest molecule alkyl-bridged methoxy-tetraphenylethylene-phenylpyridines derivative, cucurbit[n]uril (n = 7, 8) and β-cyclodextrin modified hyaluronic acid. The high binding affinity of cucurbituril to guest molecules in various stoichiometric ratios not only regulates the topological morphology of supramolecular assembly but also induces different phosphorescence emissions. Varying from the spherical nanoparticles and nanorods for binary assemblies, three-dimensional nanoplate is obtained by the ternary co-assembly of guest with cucurbit[7]uril/cucurbit[8]uril, accompanying enhanced phosphorescence at 540 nm. Uncommonly, the secondary assembly of β-cyclodextrin modified hyaluronic acid and ternary assembly activates a single intramolecular phosphorescence resonance energy transfer process derived from phenyl pyridines unit to methoxy-tetraphenylethylene function group, enabling a near-infrared delayed fluorescence at 700 nm, which ultimately applied to mitochondrial targeted imaging for cancer cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wang X, Han K, Li J, Jia X, Li C. Pillar[5]arene-neutral guest recognition based supramolecular alternating copolymer containing [c2] daisy chain and bis-pillar[5]arene units. Polym. Chem. 2013;4:3998–4003. doi: 10.1039/c3py00462g. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

