Blocking Oncostatin M receptor abrogates STAT3 mediated integrin signaling and overcomes chemoresistance in ovarian cancer
- PMID: 38839865
- PMCID: PMC11153533
- DOI: 10.1038/s41698-024-00593-y
Blocking Oncostatin M receptor abrogates STAT3 mediated integrin signaling and overcomes chemoresistance in ovarian cancer
Abstract
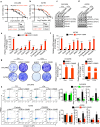
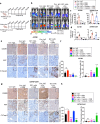
Chemotherapy such as cisplatin is widely used to treat ovarian cancer either before or after surgical debulking. However, cancer relapse due to chemotherapy resistance is a major challenge in the treatment of ovarian cancer. The underlying mechanisms related to chemotherapy resistance remain largely unclear. Therefore, identification of effective therapeutic strategies is urgently needed to overcome therapy resistance. Transcriptome-based analysis, in vitro studies and functional assays identified that cisplatin-resistant ovarian cancer cells express high levels of OSMR compared to cisplatin sensitive cells. Furthermore, OSMR expression associated with a module of integrin family genes and predominantly linked with integrin αV (ITGAV) and integrin β3 (ITGB3) for cisplatin resistance. Using ectopic expression and knockdown approaches, we proved that OSMR directly regulates ITGAV and ITGB3 gene expression through STAT3 activation. Notably, targeting OSMR using anti-OSMR human antibody inhibited the growth and metastasis of ovarian cancer cells and sensitized cisplatin treatment. Taken together, our results underscore the pivotal role of OSMR as a requirement for cisplatin resistance in ovarian cancer. Notably, OSMR fostered the expression of a distinct set of integrin genes, which in turn resulted into a crosstalk between OSMR and integrins for signaling activation that is critical for cisplatin resistance. Therefore, targeting OSMR emerges as a promising and viable strategy to reverse cisplatin-resistance in ovarian cancer.
© 2024. The Author(s).
Conflict of interest statement
P.C.-R, S.P., A.G., Z.K., N.Z., and Z.A. are inventors of a patent PCT/US2022/074276 on OSMR antibody discovery and therapeutic uses. The other authors have no competing interests to declare.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

