Genome-wide meta-analyses of restless legs syndrome yield insights into genetic architecture, disease biology and risk prediction
- PMID: 38839884
- PMCID: PMC11176086
- DOI: 10.1038/s41588-024-01763-1
Genome-wide meta-analyses of restless legs syndrome yield insights into genetic architecture, disease biology and risk prediction
Abstract
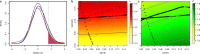
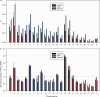
Restless legs syndrome (RLS) affects up to 10% of older adults. Their healthcare is impeded by delayed diagnosis and insufficient treatment. To advance disease prediction and find new entry points for therapy, we performed meta-analyses of genome-wide association studies in 116,647 individuals with RLS (cases) and 1,546,466 controls of European ancestry. The pooled analysis increased the number of risk loci eightfold to 164, including three on chromosome X. Sex-specific meta-analyses revealed largely overlapping genetic predispositions of the sexes (rg = 0.96). Locus annotation prioritized druggable genes such as glutamate receptors 1 and 4, and Mendelian randomization indicated RLS as a causal risk factor for diabetes. Machine learning approaches combining genetic and nongenetic information performed best in risk prediction (area under the curve (AUC) = 0.82-0.91). In summary, we identified targets for drug development and repurposing, prioritized potential causal relationships between RLS and relevant comorbidities and risk factors for follow-up and provided evidence that nonlinear interactions are likely relevant to RLS risk prediction.
© 2024. The Author(s).
Conflict of interest statement
The funders of the study had no role in conceptualization, design, data collection, analysis, the decision to publish or preparation of the manuscript. J.W., B.S., K.O. and C.Z. have filed a patent application (WO2021185936A1). Z.K.W. serves as PI or co-PI on Biohaven Pharmaceuticals (BHV4157-206), Neuraly (NLY01-PD-1) and Vigil Neuroscience (VGL101-01.002, VGL101-01.201, PET tracer development protocol, and CSF1R biomarker and repository project) grants. Z.K.W. serves as co-PI of the Mayo Clinic APDA Center for Advanced Research and as an external advisory board member for Vigil Neuroscience. W.P. has received honoraria as a speaker from Philips and MediPark Clinic and as a consultant from Abbott and Precisis. J. Danesh serves on scientific advisory boards for AstraZeneca, Novartis and the UK Biobank and has received multiple grants from academic, charitable and industry sources outside of the submitted work. A.S.B. reports institutional grants from AstraZeneca, Bayer, Biogen, BioMarin, Bioverativ, Novartis, Regeneron and Sanofi. D.A.H., N.A.F., P.N. and members of the 23andMe Research Team are employed by and hold stock or stock options in 23andMe. Authors affiliated with deCODE Genetics/Amgen declare competing financial interests as employees. The other authors declare no competing interests.
Figures









References
-
- Allen RP, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. - PubMed
-
- Manconi M, et al. Restless legs syndrome. Nat. Rev. Dis. Primers. 2021;7:80. - PubMed
-
- Allen RP, et al. Restless legs syndrome/Willis–Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—history, rationale, description, and significance. Sleep Med. 2014;15:860–873. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

