Deficiency of CAMSAP2 impairs olfaction and the morphogenesis of mitral cells
- PMID: 38839944
- PMCID: PMC11239855
- DOI: 10.1038/s44319-024-00166-x
Deficiency of CAMSAP2 impairs olfaction and the morphogenesis of mitral cells
Abstract
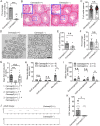
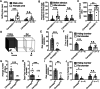
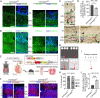
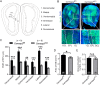
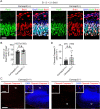
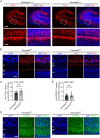
In developing olfactory bulb (OB), mitral cells (MCs) remodel their dendrites to establish the precise olfactory circuit, and these circuits are critical for individuals to sense odors and elicit behaviors for survival. However, how microtubules (MTs) participate in the process of dendritic remodeling remains elusive. Here, we reveal that calmodulin-regulated spectrin-associated proteins (CAMSAPs), a family of proteins that bind to the minus-end of the noncentrosomal MTs, play a crucial part in the development of MC dendrites. We observed that Camsap2 knockout (KO) males are infertile while the reproductive tract is normal. Further study showed that the infertility was due to the severe defects of mating behavior in male mice. Besides, mice with loss-of-function displayed defects in the sense of smell. Furthermore, we found that the deficiency of CAMSAP2 impairs the classical morphology of MCs, and the CAMSAP2-dependent dendritic remodeling process is responsible for this defect. Thus, our findings demonstrate that CAMSAP2 plays a vital role in regulating the development of MCs.
Keywords: CAMSAP2; Mitral Cell; Morphogenesis; Odor-dependent Behaviors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- 32100760/MOST | National Natural Science Foundation of China (NSFC)
- 31930025/MOST | National Natural Science Foundation of China (NSFC)
- 32270736/MOST | National Natural Science Foundation of China (NSFC)
- 2018YFA0801104/MOST | National Key Research and Development Program of China (NKPs)
- 2021YFA0804802/MOST | National Key Research and Development Program of China (NKPs)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

