Myelin plasticity in the ventral tegmental area is required for opioid reward
- PMID: 38839962
- PMCID: PMC11186775
- DOI: 10.1038/s41586-024-07525-7
Myelin plasticity in the ventral tegmental area is required for opioid reward
Abstract
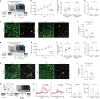
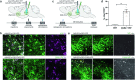
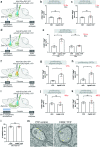
All drugs of abuse induce long-lasting changes in synaptic transmission and neural circuit function that underlie substance-use disorders1,2. Another recently appreciated mechanism of neural circuit plasticity is mediated through activity-regulated changes in myelin that can tune circuit function and influence cognitive behaviour3-7. Here we explore the role of myelin plasticity in dopaminergic circuitry and reward learning. We demonstrate that dopaminergic neuronal activity-regulated myelin plasticity is a key modulator of dopaminergic circuit function and opioid reward. Oligodendroglial lineage cells respond to dopaminergic neuronal activity evoked by optogenetic stimulation of dopaminergic neurons, optogenetic inhibition of GABAergic neurons, or administration of morphine. These oligodendroglial changes are evident selectively within the ventral tegmental area but not along the axonal projections in the medial forebrain bundle nor within the target nucleus accumbens. Genetic blockade of oligodendrogenesis dampens dopamine release dynamics in nucleus accumbens and impairs behavioural conditioning to morphine. Taken together, these findings underscore a critical role for oligodendrogenesis in reward learning and identify dopaminergic neuronal activity-regulated myelin plasticity as an important circuit modification that is required for opioid reward.
© 2024. The Author(s).
Conflict of interest statement
R.C.M. is now on leave from Stanford, functioning as the Chief Scientific Officer at Bayshore Global Management. He is on the scientific advisory boards of MapLight Therapeutics, MindMed, Bright Minds Biosciences and Aelis Farma. M.M. holds equity in MapLight Therapeutics and CARGO Biosciences. The remaining authors declare no competing interests.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

