Descending networks transform command signals into population motor control
- PMID: 38839968
- PMCID: PMC11186778
- DOI: 10.1038/s41586-024-07523-9
Descending networks transform command signals into population motor control
Abstract
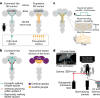
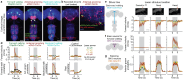
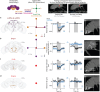
To convert intentions into actions, movement instructions must pass from the brain to downstream motor circuits through descending neurons (DNs). These include small sets of command-like neurons that are sufficient to drive behaviours1-the circuit mechanisms for which remain unclear. Here we show that command-like DNs in Drosophila directly recruit networks of additional DNs to orchestrate behaviours that require the active control of numerous body parts. Specifically, we found that command-like DNs previously thought to drive behaviours alone2-4 in fact co-activate larger populations of DNs. Connectome analyses and experimental manipulations revealed that this functional recruitment can be explained by direct excitatory connections between command-like DNs and networks of interconnected DNs in the brain. Descending population recruitment is necessary for behavioural control: DNs with many downstream descending partners require network co-activation to drive complete behaviours and drive only simple stereotyped movements in their absence. These DN networks reside within behaviour-specific clusters that inhibit one another. These results support a mechanism for command-like descending control in which behaviours are generated through the recruitment of increasingly large DN networks that compose behaviours by combining multiple motor subroutines.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













Similar articles
-
Distributed control of motor circuits for backward walking in Drosophila.Nat Commun. 2020 Dec 2;11(1):6166. doi: 10.1038/s41467-020-19936-x. Nat Commun. 2020. PMID: 33268800 Free PMC article.
-
Descending neuron population dynamics during odor-evoked and spontaneous limb-dependent behaviors.Elife. 2022 Oct 26;11:e81527. doi: 10.7554/eLife.81527. Elife. 2022. PMID: 36286408 Free PMC article.
-
MDN brain descending neurons coordinately activate backward and inhibit forward locomotion.Elife. 2018 Aug 2;7:e38554. doi: 10.7554/eLife.38554. Elife. 2018. PMID: 30070205 Free PMC article.
-
A connectome and analysis of the adult Drosophila central brain.Elife. 2020 Sep 7;9:e57443. doi: 10.7554/eLife.57443. Elife. 2020. PMID: 32880371 Free PMC article.
-
Of what use is connectomics? A personal perspective on the Drosophila connectome.J Exp Biol. 2018 May 20;221(Pt 10):jeb164954. doi: 10.1242/jeb.164954. J Exp Biol. 2018. PMID: 29784759 Review.
Cited by
-
Comparative connectomics of the descending and ascending neurons of the Drosophila nervous system: stereotypy and sexual dimorphism.bioRxiv [Preprint]. 2024 Jun 28:2024.06.04.596633. doi: 10.1101/2024.06.04.596633. bioRxiv. 2024. PMID: 38895426 Free PMC article. Preprint.
-
Biomaterials for neuroengineering: applications and challenges.Regen Biomater. 2025 Feb 21;12:rbae137. doi: 10.1093/rb/rbae137. eCollection 2025. Regen Biomater. 2025. PMID: 40007617 Free PMC article. Review.
-
Comparative connectomics of Drosophila descending and ascending neurons.Nature. 2025 Jul;643(8070):158-172. doi: 10.1038/s41586-025-08925-z. Epub 2025 Apr 30. Nature. 2025. PMID: 40307549 Free PMC article.
-
Connectomic analysis of taste circuits in Drosophila.Sci Rep. 2025 Feb 12;15(1):5278. doi: 10.1038/s41598-025-89088-9. Sci Rep. 2025. PMID: 39939650 Free PMC article.
-
Inhibitory circuit motifs in Drosophila larvae generate motor program diversity and variability.PLoS Biol. 2025 Apr 21;23(4):e3003094. doi: 10.1371/journal.pbio.3003094. eCollection 2025 Apr. PLoS Biol. 2025. PMID: 40258087 Free PMC article.
References
-
- Lassek AM, Rasmussen GL. A comparative fiber and numerical analysis of the pyramidal tract. J. Comp. Neurol. 1940;72:417–428. doi: 10.1002/cne.900720209. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

