Functional diversity among cardiolipin binding sites on the mitochondrial ADP/ATP carrier
- PMID: 38839991
- PMCID: PMC11251061
- DOI: 10.1038/s44318-024-00132-2
Functional diversity among cardiolipin binding sites on the mitochondrial ADP/ATP carrier
Abstract
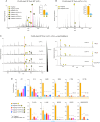
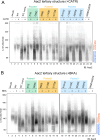
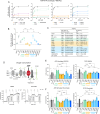
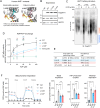
Lipid-protein interactions play a multitude of essential roles in membrane homeostasis. Mitochondrial membranes have a unique lipid-protein environment that ensures bioenergetic efficiency. Cardiolipin (CL), the signature mitochondrial lipid, plays multiple roles in promoting oxidative phosphorylation (OXPHOS). In the inner mitochondrial membrane, the ADP/ATP carrier (AAC in yeast; adenine nucleotide translocator, ANT in mammals) exchanges ADP and ATP, enabling OXPHOS. AAC/ANT contains three tightly bound CLs, and these interactions are evolutionarily conserved. Here, we investigated the role of these buried CLs in AAC/ANT using a combination of biochemical approaches, native mass spectrometry, and molecular dynamics simulations. We introduced negatively charged mutations into each CL-binding site of yeast Aac2 and established experimentally that the mutations disrupted the CL interactions. While all mutations destabilized Aac2 tertiary structure, transport activity was impaired in a binding site-specific manner. Additionally, we determined that a disease-associated missense mutation in one CL-binding site in human ANT1 compromised its structure and transport activity, resulting in OXPHOS defects. Our findings highlight the conserved significance of CL in AAC/ANT structure and function, directly tied to specific lipid-protein interactions.
Keywords: Cardiolipin; Lipid–Protein Interaction; Membrane Transport; Mitochondria; Oxidative Phosphorylation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Conserved cardiolipin-mitochondrial ADP/ATP carrier interactions assume distinct structural and functional roles that are clinically relevant.bioRxiv [Preprint]. 2023 May 6:2023.05.05.539595. doi: 10.1101/2023.05.05.539595. bioRxiv. 2023. Update in: EMBO J. 2024 Jul;43(14):2979-3008. doi: 10.1038/s44318-024-00132-2. PMID: 37205478 Free PMC article. Updated. Preprint.
References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
-
- Acoba MG, Alpergin ESS, Renuse S, Fernández-del-Río L, Lu Y-W, Khalimonchuk O, Clarke CF, Pandey A, Wolfgang MJ, Claypool SM. The mitochondrial carrier SFXN1 is critical for complex III integrity and cellular metabolism. Cell Rep. 2021;34:108869. doi: 10.1016/j.celrep.2021.108869. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL108882/HL/NHLBI NIH HHS/United States
- Award ID: 828058/American Heart Association (AHA)
- 15PRE24480066/American Heart Association (AHA)
- T32GM007445/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- Award ID: 828058/Barth Syndrome Foundation (BSF)
- NIF\R1\192285/Office of the Royal Society
- R01HL165729/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- T32GM136577/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R35GM119762/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- T32 GM136577/GM/NIGMS NIH HHS/United States
- R35 GM119762/GM/NIGMS NIH HHS/United States
- 10PRE3280013/American Heart Association (AHA)
- T32 GM007445/GM/NIGMS NIH HHS/United States
- R01 HL165729/HL/NHLBI NIH HHS/United States
- R01HL108882/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- NIF\R1\181108/Royal Society (The Royal Society)
LinkOut - more resources
Full Text Sources

