Whole-body replacement of larval myofibers generates permanent adult myofibers in zebrafish
- PMID: 38839992
- PMCID: PMC11294464
- DOI: 10.1038/s44318-024-00136-y
Whole-body replacement of larval myofibers generates permanent adult myofibers in zebrafish
Abstract
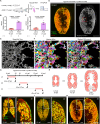
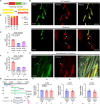
Drastic increases in myofiber number and size are essential to support vertebrate post-embryonic growth. However, the collective cellular behaviors that enable these increases have remained elusive. Here, we created the palmuscle myofiber tagging and tracking system for in toto monitoring of the growth and fates of ~5000 fast myofibers in developing zebrafish larvae. Through live tracking of individual myofibers within the same individuals over extended periods, we found that many larval myofibers readily dissolved during development, enabling the on-site addition of new and more myofibers. Remarkably, whole-body surveillance of multicolor-barcoded myofibers further unveiled a gradual yet extensive elimination of larval myofiber populations, resulting in near-total replacement by late juvenile stages. The subsequently emerging adult myofibers are not only long-lasting, but also morphologically and functionally distinct from the larval populations. Furthermore, we determined that the elimination-replacement process is dependent on and driven by the autophagy pathway. Altogether, we propose that the whole-body replacement of larval myofibers is an inherent yet previously unnoticed process driving organismic muscle growth during vertebrate post-embryonic development.
Keywords: Fast Myofiber; Live Imaging; Post-embryonic Growth; Zebrafish.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











References
-
- Bindels DS, Haarbosch L, van Weeren L, Postma M, Wiese KE, Mastop M, Aumonier S, Gotthard G, Royant A, Hink MA, Gadella Jr TW (2017) mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat Methods 14:53–56 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

