CD36 deletion prevents white matter injury by modulating microglia polarization through the Traf5-MAPK signal pathway
- PMID: 38840180
- PMCID: PMC11155181
- DOI: 10.1186/s12974-024-03143-2
CD36 deletion prevents white matter injury by modulating microglia polarization through the Traf5-MAPK signal pathway
Abstract
Background: White matter injury (WMI) represents a significant etiological factor contributing to neurological impairment subsequent to Traumatic Brain Injury (TBI). CD36 receptors are recognized as pivotal participants in the pathogenesis of neurological disorders, including stroke and spinal cord injury. Furthermore, dynamic fluctuations in the phenotypic polarization of microglial cells have been intimately associated with the regenerative processes within the injured tissue following TBI. Nevertheless, there is a paucity of research addressing the impact of CD36 receptors on WMI and microglial polarization. This investigation aims to elucidate the functional role and mechanistic underpinnings of CD36 in modulating microglial polarization and WMI following TBI.
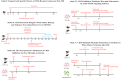
Methods: TBI models were induced in murine subjects via controlled cortical impact (CCI). The spatiotemporal patterns of CD36 expression were examined through quantitative polymerase chain reaction (qPCR), Western blot analysis, and immunofluorescence staining. The extent of white matter injury was assessed via transmission electron microscopy, Luxol Fast Blue (LFB) staining, and immunofluorescence staining. Transcriptome sequencing was employed to dissect the molecular mechanisms underlying CD36 down-regulation and its influence on white matter damage. Microglial polarization status was ascertained using qPCR, Western blot analysis, and immunofluorescence staining. In vitro, a Transwell co-culture system was employed to investigate the impact of CD36-dependent microglial polarization on oligodendrocytes subjected to oxygen-glucose deprivation (OGD).
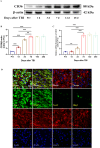
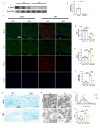
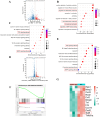
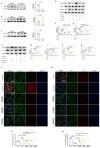
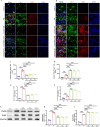
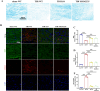
Results: Western blot and qPCR analyses revealed that CD36 expression reached its zenith at 7 days post-TBI and remained sustained at this level thereafter. Immunofluorescence staining exhibited robust CD36 expression in astrocytes and microglia following TBI. Genetic deletion of CD36 ameliorated TBI-induced white matter injury, as evidenced by a reduced SMI-32/MBP ratio and G-ratio. Transcriptome sequencing unveiled differentially expressed genes enriched in processes linked to microglial activation, regulation of neuroinflammation, and the TNF signaling pathway. Additionally, bioinformatics analysis pinpointed the Traf5-p38 axis as a critical signaling pathway. In vivo and in vitro experiments indicated that inhibition of the CD36-Traf5-MAPK axis curtailed microglial polarization toward the pro-inflammatory phenotype. In a Transwell co-culture system, BV2 cells treated with LPS + IFN-γ exacerbated the damage of post-OGD oligodendrocytes, which could be rectified through CD36 knockdown in BV2 cells.
Conclusions: This study illuminates that the suppression of CD36 mitigates WMI by constraining microglial polarization towards the pro-inflammatory phenotype through the down-regulation of the Traf5-MAPK signaling pathway. Our findings present a potential therapeutic strategy for averting neuroinflammatory responses and ensuing WMI damage resulting from TBI.
Keywords: CD36; Microglial polarization; Traumatic brain injury; White matter injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

