Effectiveness of janus kinase inhibitors in relapsing giant cell arteritis in real-world clinical practice and review of the literature
- PMID: 38840219
- PMCID: PMC11151571
- DOI: 10.1186/s13075-024-03314-9
Effectiveness of janus kinase inhibitors in relapsing giant cell arteritis in real-world clinical practice and review of the literature
Abstract
Background: A substantial proportion of patients with giant cell arteritis (GCA) relapse despite standard therapy with glucocorticoids, methotrexate and tocilizumab. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway is involved in the pathogenesis of GCA and JAK inhibitors (JAKi) could be a therapeutic alternative. We evaluated the effectiveness of JAKi in relapsing GCA patients in a real-world setting and reviewed available literature.
Methods: Retrospective analysis of GCA patients treated with JAKi for relapsing disease at thirteen centers in Spain and one center in United States (01/2017-12/2022). Outcomes assessed included clinical remission, complete remission and safety. Clinical remission was defined as the absence of GCA signs and symptoms regardless of the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values. Complete remission was defined as the absence of GCA signs and symptoms along with normal ESR and CRP values. A systematic literature search for other JAKi-treated GCA cases was conducted.
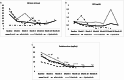
Results: Thirty-five patients (86% females, mean age 72.3) with relapsing GCA received JAKi therapy (baricitinib, n = 15; tofacitinib, n = 10; upadacitinib, n = 10). Before JAKi therapy, 22 (63%) patients had received conventional synthetic immunosuppressants (e.g., methotrexate), and 30 (86%) biologics (e.g., tocilizumab). After a median (IQR) follow-up of 11 (6-15.5) months, 20 (57%) patients achieved and maintained clinical remission, 16 (46%) patients achieved and maintained complete remission, and 15 (43%) patients discontinued the initial JAKi due to relapse (n = 11 [31%]) or serious adverse events (n = 4 [11%]). A literature search identified another 36 JAKi-treated GCA cases with clinical improvement reported for the majority of them.
Conclusions: This real-world analysis and literature review suggest that JAKi could be effective in GCA, including in patients failing established glucocorticoid-sparing therapies such as tocilizumab and methotrexate. A phase III randomized controlled trial of upadacitinib is currently ongoing (ClinicalTrials.gov ID NCT03725202).
Keywords: Baricitinib; Giant cell arteritis; Janus kinase inhibitors; Large vessel vasculitis; Tofacitinib; Upadacitinib.
© 2024. The Author(s).
Conflict of interest statement
Disclosures that might be interpreted as constituting possible conflict(s) of interest for the study: Javier Loricera had consultation fees/participation in company-sponsored speaker´s bureau from Roche, AbbVie, Galápagos, Novartis, UCB Pharma, MSD, Celgene, Astra Zeneca, and Grünenthal and received support for attending meetings and/or travel from Janssen, AbbVie, Roche, Novartis, MSD, UCB Pharma, Celgene, Lilly, Pfizer, and Galápagos. Diana Prieto-Peña has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from UCB Pharma, Novartis, Amgen, Lilly, Sanofi, and Janssen, and received support for attending meetings and/or travel from Lilly, Roche, Sanofi, Pfizer and Abbvie. Susana Romero-Yuste has received grants/recents supports from Abbvie, Lilly, Pfizer, and Galápagos. Eugenio de Miguel Research funding/consulting and conferences fees from: Abbvie, Novartis, Pfizer, Roche, Janssen, Lilly, MSD, BMS, UCB, Grünenthal, and Sanofi, and received support for attending meetings and/or travel from Abbvie, Pfizer, Sanofi, and Grünenthal. Iván Ferraz-Amaro would like to acknowledge that he has received grants/research supports from Abbvie, MSD, Janssen, and Roche, as well as consultation fees from company sponsored speakers bureaus associated with Abbvie, Pfizer, Roche, Sanofi, Celgene, and MSD, and received support for attending meetings and/or travel from Abbvie, MSD, Janssen, Pfizer, Roche, Sanofi, and Celgene. Eva Galíndez-Agirregoikoa had consultation fees/participation in company-sponsored speaker´s bureau from Lilly, Janssen, Abbvie, and Amgen, and received support for attenting meetings and/or travel from Lilly, Abbvie, and Pfizer. Ismael González-Fernández had consultation fees/participation in company-sponsored speaker´s bureau from Novartis, Janssen, Grunenthal, MSD, and Theramex, and received support for attending meetings and/or travel from Janssen, and MSD. Ana Urruticoechea-Arana had received research grants, fees for consultancies or presentations and participated in medical meetings or courses from Abbvie, Galápagos, UCB, Pfizer, MSD, Novartis, GSK, Hanssen, and Amgen, and participated on a data safety monitoring board or advisory board GSK. Ángel Ramos-Calvo had consultation fees/participation in company-sponsored speaker´s bureau from Amgen, UCB, and Galápagos, and received support for attending meetings and/or travel from Pfizer. Fernando López-Gutiérrez received support for attending meetings and/or travel from Janssen, Abbvie, Roche, Novartis, MSD, UCB Pharma, Celgene, Lilly, Pfizer, and Galápagos. Santos Castañeda has received research supports from MSD, and Pfizer and had consultation fees/participation in company-sponsored speaker’s bureau from BMS, Eli-Lilly, MSD, Roche, and UCB, and received support for attending meetings and/or travel from BMS, Lilly, MSD, Roche, and UCB. Sebastian Unizony received research support from Genentech, participated in an advisory board for Abbvie, and provided consulting for Sanofi, Kiniksa, and Janssen.Ricardo Blanco received grants/research support from AbbVie, MSD, and Roche, and had consultation fees/participation in a company-sponsored speaker’s bureau from AbbVie, Pfizer, Roche, GSK, Lilly, UCB, Bristol-Myers, Novartis, Janssen, UCB and MSD, and received support for attending meetings and/or travel from AbbVie, Pfizer, Roche, GSK, Lilly, UCB, Bristol-Myers, Novartis, Janssen, UCB, and MSD.
Figures

References
-
- Loricera J, Blanco R, Hernández JL, et al. Use of positron emission tomography (PET) for the diagnosis of large-vessel vasculitis. Rev Esp Med Nucl Imagen Mol. 2015;34:372–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

