Platelet transfusions in adult ICU patients with thrombocytopenia: A sub-study of the PLOT-ICU inception cohort study
- PMID: 38840310
- PMCID: PMC11729610
- DOI: 10.1111/aas.14467
Platelet transfusions in adult ICU patients with thrombocytopenia: A sub-study of the PLOT-ICU inception cohort study
Abstract
Background: Platelet transfusions are frequently used in the intensive care unit (ICU), but current practices including used product types, volumes, doses and effects are unknown.
Study design and methods: Sub-study of the inception cohort study 'Thrombocytopenia and Platelet Transfusions in the ICU (PLOT-ICU)', including acutely admitted, adult ICU patients with thrombocytopenia (platelet count <150 × 109/L). The primary outcome was the number of patients receiving platelet transfusion in ICU by product type. Secondary outcomes included platelet transfusion details, platelet increments, bleeding, other transfusions and mortality.
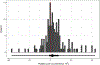
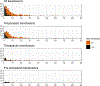
Results: Amongst 504 patients with thrombocytopenia from 43 hospitals in 10 countries in Europe and the United States, 20.8% received 565 platelet transfusions; 61.0% received pooled products, 21.9% received apheresis products and 17.1% received both with a median of 2 (interquartile range 1-4) days from admission to first transfusion. The median volume per transfusion was 253 mL (180-308 mL) and pooled products accounted for 59.1% of transfusions, however, this varied across countries. Most centres (73.8%) used fixed dosing (medians ranging from 2.0 to 3.5 × 1011 platelets/transfusion) whilst some (mainly in France) used weight-based dosing (ranging from 0.5 to 0.7 × 1011 platelets per 10 kg body weight). The median platelet count increment for a single prophylactic platelet transfusion was 2 (-1 to 8) × 109/L. Outcomes of patients with thrombocytopenia who did and did not receive platelet transfusions varied.
Conclusions: Among acutely admitted, adult ICU patients with thrombocytopenia, 20.8% received platelet transfusions in ICU of whom most received pooled products, but considerable variation was observed in product type, volumes and doses across countries. Prophylactic platelet transfusions were associated with limited increases in platelet counts.
Keywords: critical illness; intensive care unit; platelet transfusion; thrombocytopenia.
© 2024 The Author(s). Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The Department of Intensive Care at Rigshospitalet (CTA, AP, MHM, LR) has received funding for other research projects from Sygeforsikringen ‘danmark’, and the Novo Nordisk Foundation. FP has received honoraria for consulting and lectures from Gilead and an institutional grant from Alexion Pharma. AP has received honorarium from Novartis for participation in an advisory board. EA has received research grants from MSD Avenir and Alexion and honoraria for lectures from Alexion, Sanofi and Pfizer. AVDL has received honoraria from Sanofi for participation in an advisory board. PC has received consulting fees from Sanofi, Gilead, Alexion and Janssen and honoraria for lectures from Merck Sharp & Dohme, Gilead, Alexion and Pfizer. PP has received consulting fees from Sanofi and Gilead and honoraria from Merck Sharp & Dohme, Gilead, Mundipharma and Pfizer for academic and educational work. EC received fees for lectures and conference talks and had travel and accommodation expenses related to attending scientific meetings covered by Gilead, Shionogi B.V. and SanofiGenzyme. ARH has received honoraria from Pfizer for lectures. MHB has received consulting fees from AM-pharma and Inotrem. CL has received consulting fees from Gilead. MS has received honoraria for lectures and consulting from CSL Behring, Alexion AstraZeneca Rare Disease and Takeda. KS has received consulting fees from Paion. None of these had any relation to the current work.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

