Evolutionary adaptations generally reverse phenotypic plasticity to restore ancestral phenotypes during new environment adaptation in cattle
- PMID: 38840586
- PMCID: PMC11150418
- DOI: 10.1002/ece3.11489
Evolutionary adaptations generally reverse phenotypic plasticity to restore ancestral phenotypes during new environment adaptation in cattle
Abstract
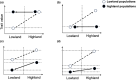
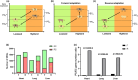
Phenotype plasticity and evolution adaptations are the two main ways in which allow populations to deal with environmental changes, but the potential relationship between them remains controversial. Using a reciprocal transplant approach with cattle adapted to the Tibetan Plateau and adjacent lowlands, we aim to investigate the relative contributions of evolutionary processes and phenotypic plasticity in driving both phenotypic and transcriptomic changes under natural conditions. We observed that while numerous genetic transcriptomic changes were evident during the forward adaptation to highland environments, plastic changes predominantly facilitate the transformation of transcriptomes into a preferred state when Tibetan cattle are reintroduced to lowland habitats. Genes with ancestral plasticity are generally reversed by evolutionary adaptations and show a closer expression level to the ancestral stage in evolved Tibetan cattle. A similar trend was also observed at the phenotypes level, with a majority of biochemical and hemorheology phenotypes showing a tendency to revert to their ancestral patterns, suggesting the restoration of ancestral expression levels is a widespread evolutionary trend during adaptation. The findings of our study contribute to the debate regarding the relative contributions of plasticity and genetic changes in mammal environment adaptation. Furthermore, we highlight that the restoration of ancestral phenotypes represents a general pattern in cattle new environment adaptation.
Keywords: cattle; environment adaptation; gene expression; phenotypic plasticity.
© 2024 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ajmone‐Marsan, P. , Garcia, J. F. , & Lenstra, J. A. (2010). On the origin of cattle: How aurochs became cattle and colonized the world. Evolutionary Anthropology, 19(4), 148–157.
-
- Baythavong, B. S. (2011). Linking the spatial scale of environmental variation and the evolution of phenotypic plasticity: Selection favors adaptive plasticity in fine‐grained environments. The American Naturalist, 178, 75–87. - PubMed
-
- Chen, F. H. , Dong, G. H. , Zhang, D. J. , Liu, X. Y. , Jia, X. , An, C. B. , Ma, M. M. , Xie, Y. W. , Barton, L. , Ren, X. Y. , Zhao, Z. J. , Wu, X. H. , & Jones, M. K. (2015). Agriculture facilitated permanent human occupation of the Tibetan Plateau after 3600 B.P. Science, 347(6219), 248–250. - PubMed
LinkOut - more resources
Full Text Sources

