Adsorption dynamics of Cd2+(aq) on microwave-synthetized pristine biochar from cocoa pod husk: Green, experimental, and DFT approaches
- PMID: 38840843
- PMCID: PMC11152673
- DOI: 10.1016/j.isci.2024.109958
Adsorption dynamics of Cd2+(aq) on microwave-synthetized pristine biochar from cocoa pod husk: Green, experimental, and DFT approaches
Abstract
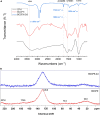
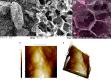
Biochar obtained via microwave-assisted pyrolysis (MAP) at 720 W and 15 min from cocoa pod husk (CPH) is an efficient adsorbent of Cd2+(aq). Biochar of residual biomass of CPH (BCCPH) possesses favorable physicochemical and morphological properties, featuring a modest surface area yet a suitable porous structure. Adsorption, predominantly governed by physisorption, is influenced by the oxygen-containing active sites (-COOR, -C(R)O, and -CH2OR; R = H, alkyl). CdCO3 formation occurs during adsorption. Experimental data were well-fitted into various kinetic models for a broad understanding of the sorption process. Langmuir model indicates a maximum adsorption capacity of 14.694 mg/g. The thermodynamic study confirms the spontaneous and endothermic sorption. Studies at the molecular level have revealed that the Cd2+ ion tends to bind to surface aromatic carbon atoms. This sustainable approach produces BCCPH via MAP as a solution for waste transformation into water-cleaning materials.
Keywords: Environmental management; Soil chemistry; Soil science.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Effect of Pyrolysis Temperature on the Sorption of Cd(II) and Se(IV) by Rice Husk Biochar.Plants (Basel). 2022 Nov 25;11(23):3234. doi: 10.3390/plants11233234. Plants (Basel). 2022. PMID: 36501273 Free PMC article.
-
N-doped and activated porous biochar derived from cocoa shell for removing norfloxacin from aqueous solution: Performance assessment and mechanism insight.Environ Res. 2022 Nov;214(Pt 3):113951. doi: 10.1016/j.envres.2022.113951. Epub 2022 Aug 15. Environ Res. 2022. PMID: 35981615
-
Efficient removal of Cd(II) from aqueous solution by pinecone biochar: Sorption performance and governing mechanisms.Environ Pollut. 2020 Oct;265(Pt A):115001. doi: 10.1016/j.envpol.2020.115001. Epub 2020 Jun 12. Environ Pollut. 2020. PMID: 32563143
-
Effect of magnetic field on the removal of copper from aqueous solution using activated carbon derived from rice husk.Environ Sci Pollut Res Int. 2022 Mar;29(14):20017-20034. doi: 10.1007/s11356-020-12158-0. Epub 2021 Jan 4. Environ Sci Pollut Res Int. 2022. PMID: 33394433
-
Cocoa by-products: A comprehensive review on potential uses, waste management, and emerging green technologies for cocoa pod husk utilization.Heliyon. 2024 Jul 31;10(16):e35537. doi: 10.1016/j.heliyon.2024.e35537. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220910 Free PMC article. Review.
References
-
- Suciu N.A., De Vivo R., Rizzati N., Capri E. Cd content in phosphate fertilizer: Which potential risk for the environment and human health? Curr. Opin. Environ. Sci. Health. 2022;30 doi: 10.1016/j.coesh.2022.100392. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

