The efficacy of cercarial antigen loaded on nanoparticles as a potential vaccine candidate in Schistosoma mansoni-infected mice
- PMID: 38840868
- PMCID: PMC11147980
- DOI: 10.1007/s12639-024-01677-z
The efficacy of cercarial antigen loaded on nanoparticles as a potential vaccine candidate in Schistosoma mansoni-infected mice
Abstract
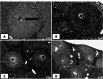
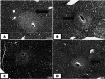
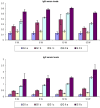
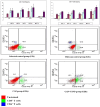
Schistosomiasis is one of the most common causes of morbidity and mortality from parasitic diseases. Mass treatment has proven to be insufficient because of repeated infection after treatment and the appearance of strains resistant to drug therapy. Hence, immunization is a new approach to control the disease and limit the pathological consequences of schistosomiasis. To evaluate the prophylactic effect of Cercarial antigen (CAP) loaded on chitosan nanoparticles (CSNPs) as a potential vaccine against Schistosoma mansoni-infected mice. 130 mice divided into 2 groups were used: Group I: Control groups (50 mice) subdivided into subgroup Ia (10 mice): Non-infected mice (normal control), subgroup Ib (20 mice): Schistosoma infected mice (infected control) and subgroup Ic (20 mice): Non-infected mice receiving NPs only. Group II: Vaccinated group (80 mice) subdivided equally into subgroup IIa (CAP): Received cercarial antigen and subgroup IIb (CAP + CSNP): Received cercarial antigen loaded on chitosan NPs then both vaccinated groups were infected with S. mansoni 3 weeks following the initial vaccination dose. CAP + CSNP and CAP groups showed significant reduction in adult worms count, hepatic egg count, hepatic granulomas number and size in comparison to the infected control group. Elevation of serum IgG and IgM levels, CD4+ and CD8+ T cell frequencies, IL-4, IL-10 and INF-γ levels was more significant in CAP + CSNP group than CAP group. CAP + CSNP is a promising new preparation of Schistosomal antigens that gave better results than immunization with CAP alone. CSNPs enhanced the immune and protective effect of CAP as validated by parasitological, histopathological and immunohistochemical studies.
Keywords: CAP; Chitosan nanoparticles; Flow cytometry; Immunization; Schistosomiasis mansoni.
© Indian Society for Parasitology 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing interestsThe authors have no relevant financial or non-financial interests to disclose.
Figures




References
-
- Ahmad G, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF, et al. Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis. 2011;204:1437–1449. doi: 10.1093/infdis/jir545. - DOI - PMC - PubMed
-
- Ahmad G, Zhang W, Torben W, Haskins C, Diggs S, Noor Z, et al. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res. 2009;105:1767–1777. doi: 10.1007/s00436-009-1646-z. - DOI - PMC - PubMed
-
- Alves CC, Araujo N, Bernardes WPOS, Mendes MM, Oliveira SC, Fonseca CT. A strong humoral immune response induced by a vaccine formulation containing rSm29 adsorbed to Alum is associated with protection against Schistosoma mansoni reinfection in mice. Front Immunol. 2018;9:1–12. doi: 10.3389/fimmu.2018.02488. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
