Carbonate precipitation and phosphate trapping by microbialite isolates from an alkaline insular lake (Bagno dell'Acqua, Pantelleria Island, Italy)
- PMID: 38841062
- PMCID: PMC11150794
- DOI: 10.3389/fmicb.2024.1391968
Carbonate precipitation and phosphate trapping by microbialite isolates from an alkaline insular lake (Bagno dell'Acqua, Pantelleria Island, Italy)
Abstract
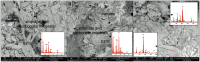
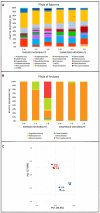
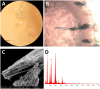
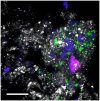
The Bagno dell'Acqua lake is characterized by CO2 emissions, alkaline waters (pH = 9) and Eh values which indicate strongly oxidizing conditions. A typical feature of the lake is the presence of actively growing microbialites rich in calcium carbonates and silica precipitates. Mineralogy, petrography and morphology analyses of the microbialites were coupled with the analysis of the microbial community, combining molecular and cultivation approaches. The DNA sequencing revealed distinct patterns of microbial diversity, showing pronounced differences between emerged and submerged microbialite, with the upper layer of emerged samples exhibiting the most distinctive composition, both in terms of prokaryotes and eukaryotes. In particular, the most representative phyla in the microbial community were Proteobacteria, Actinobacteriota, and Bacteroidota, while Cyanobacteria were present only with an average of 5%, with the highest concentration in the submerged intermediate layer (12%). The role of microorganisms in carbonate mineral formation was clearly demonstrated as most of the isolates were able to precipitate calcium carbonate and five of them were characterized at molecular level. Interestingly, when microbial isolates were cultivated only in filtered water, the precipitation of hazenite was observed (up to 85%), opening new prospective in P (phosphate) recovery from P depleted environments.
Keywords: alkaline lake; biomineralization; hazenite; microbialites; pantelleria; phosphate trapping.
Copyright © 2024 Mazzoni, Piacentini, Di Bella, Aldega, Perinelli, Conte, Ingrassia, Ruspandini, Bonfanti, Caraba, Falese, Chiocci and Fazi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Abdel-Azeem A. M., Salem F. M., Abdel-Azeem M. A., Nafady N. A., Mohesien M. T., Soliman E. A. (2016). “Biodiversity of the genus Aspergillus in different habitats,” in New and Future Developments in Microbial Biotechnology and Bioengineering (Amsterdam: Elsevier; ), 3–28.
-
- Apprill A., McNally S., Parsons R., Weber L. (2015). Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquatic Microb. Ecol. 75, 129–137. 10.3354/ame01753 - DOI
LinkOut - more resources
Full Text Sources

