Distribution-informed and wavelength-flexible data-driven photoacoustic oximetry
- PMID: 38841431
- PMCID: PMC11151660
- DOI: 10.1117/1.JBO.29.S3.S33303
Distribution-informed and wavelength-flexible data-driven photoacoustic oximetry
Abstract
Significance: Photoacoustic imaging (PAI) promises to measure spatially resolved blood oxygen saturation but suffers from a lack of accurate and robust spectral unmixing methods to deliver on this promise. Accurate blood oxygenation estimation could have important clinical applications from cancer detection to quantifying inflammation.
Aim: We address the inflexibility of existing data-driven methods for estimating blood oxygenation in PAI by introducing a recurrent neural network architecture.
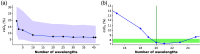
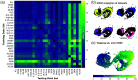
Approach: We created 25 simulated training dataset variations to assess neural network performance. We used a long short-term memory network to implement a wavelength-flexible network architecture and proposed the Jensen-Shannon divergence to predict the most suitable training dataset.
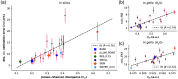
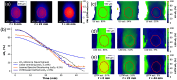
Results: The network architecture can flexibly handle the input wavelengths and outperforms linear unmixing and the previously proposed learned spectral decoloring method. Small changes in the training data significantly affect the accuracy of our method, but we find that the Jensen-Shannon divergence correlates with the estimation error and is thus suitable for predicting the most appropriate training datasets for any given application.
Conclusions: A flexible data-driven network architecture combined with the Jensen-Shannon divergence to predict the best training data set provides a promising direction that might enable robust data-driven photoacoustic oximetry for clinical use cases.
Keywords: deep learning; image processing; oximetry; quantitative imaging; simulation.
© 2024 The Authors.
Figures