IKZF1 gene deletions drive resistance to cytarabine in B-cell precursor acute lymphoblastic leukemia
- PMID: 38841778
- PMCID: PMC11609812
- DOI: 10.3324/haematol.2023.284357
IKZF1 gene deletions drive resistance to cytarabine in B-cell precursor acute lymphoblastic leukemia
Abstract
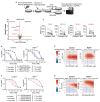
IKZF1 deletions occur in 10-15% of patients with B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and predict a poor outcome. However, the impact of IKZF1 loss on sensitivity to drugs used in contemporary treatment protocols has remained underexplored. Here we show in experimental models and in patients that loss of IKZF1 promotes resistance to cytarabine (AraC), a key component of both upfront and relapsed treatment protocols. We attribute this resistance, in part, to diminished import and incorporation of AraC due to reduced expression of the solute carrier hENT1. Moreover, we found elevated mRNA expression of Evi1, a known driver of therapy resistance in myeloid malignancies. Finally, a kinase directed CRISPR/Cas9-screen identified that inhibition of either mediator kinases CDK8/19 or casein kinase 2 can restore response to AraC. We conclude that this high-risk group of patients could benefit from alternative antimetabolites, or targeted therapies that re-sensitize leukemic cells to AraC.
Figures




References
-
- Pieters R, de Groot-Kruseman H, Van der Velden V, et al. . Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol. 2016;34(22):2591-2601. - PubMed
-
- Kuiper R, Waanders E, Van Der Velden V, et al. . IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24(7):1258-1264. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

