Epstein-Barr virus and immune status imprint the immunogenomics of non-Hodgkin lymphomas occurring in immune-suppressed environments
- PMID: 38841782
- PMCID: PMC11532699
- DOI: 10.3324/haematol.2023.284332
Epstein-Barr virus and immune status imprint the immunogenomics of non-Hodgkin lymphomas occurring in immune-suppressed environments
Abstract
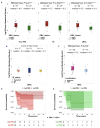
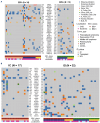
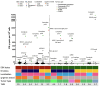
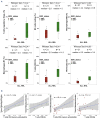
Non-Hodgkin lymphomas (NHL) commonly occur in immunodeficient patients, both those infected by human immunodeficiency virus (HIV) and those who have been transplanted, and are often driven by Epstein-Barr virus (EBV) with cerebral localization, raising the question of tumor immunogenicity, a critical issue for treatment responses. We investigated the immunogenomics of 68 lymphoproliferative disorders from 51 immunodeficient (34 post-transplant, 17 HIV+) and 17 immunocompetent patients. Overall, 72% were large B-cell lymphoma and 25% were primary central nervous system lymphoma, while 40% were EBV+. Tumor whole-exome and RNA sequencing, along with a bioinformatics pipeline allowed analysis of tumor mutational burden, tumor landscape and tumor microenvironment and prediction of tumor neoepitopes. Both tumor mutational burden (2.2 vs. 3.4/Mb, P=0.001) and numbers of neoepitopes (40 vs. 200, P=0.00019) were lower in EBV+ than in EBV- NHL, regardless of the immune status. In contrast both EBV and the immune status influenced the tumor mutational profile, with HNRNPF and STAT3 mutations observed exclusively in EBV+ and immunodeficient NHL, respectively. Peripheral blood T-cell responses against tumor neoepitopes were detected in all EBV- cases but in only half of the EBV+ ones, including responses against IgH-derived MHC-class-II restricted neoepitopes. The tumor microenvironment analysis showed higher CD8 T-cell infiltrates in EBV+ versus EBV- NHL, together with a more tolerogenic profile composed of regulatory T cells, type-M2 macrophages and an increased expression of negative immune-regulators. Our results highlight that the immunogenomics of NHL in patients with immunodeficiency primarily relies on the tumor EBV status, while T-cell recognition of tumor- and IgH-specific neoepitopes is conserved in EBV- patients, offering potential opportunities for future T-cell-based immune therapies.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

