Cross-Frequency Coupling as a Biomarker for Early Stroke Recovery
- PMID: 38842027
- PMCID: PMC11179969
- DOI: 10.1177/15459683241257523
Cross-Frequency Coupling as a Biomarker for Early Stroke Recovery
Abstract
Background: The application of neuroimaging-based biomarkers in stroke has enriched our understanding of post-stroke recovery mechanisms, including alterations in functional connectivity based on synchronous oscillatory activity across various cortical regions. Phase-amplitude coupling, a type of cross-frequency coupling, may provide additional mechanistic insight.
Objective: To determine how the phase of prefrontal cortex delta (1-3 Hz) oscillatory activity mediates the amplitude of motor cortex beta (13-20 Hz) oscillations in individual's early post-stroke.
Methods: Participants admitted to an inpatient rehabilitation facility completed resting and task-based EEG recordings and motor assessments around the time of admission and discharge along with structural neuroimaging. Unimpaired controls completed EEG procedures during a single visit. Mixed-effects linear models were performed to assess within- and between-group differences in delta-beta prefrontomotor coupling. Associations between coupling and motor status and injury were also determined.
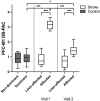
Results: Thirty individuals with stroke and 17 unimpaired controls participated. Coupling was greater during task versus rest conditions for all participants. Though coupling during affected extremity task performance decreased during hospitalization, coupling remained elevated at discharge compared to controls. Greater baseline coupling was associated with better motor status at admission and discharge and positively related to motor recovery. Coupling demonstrated both positive and negative associations with injury involving measures of lesion volume and overlap injury to anterior thalamic radiation, respectively.
Conclusions: This work highlights the utility of prefrontomotor cross-frequency coupling as a potential motor status and recovery biomarker in stroke. The frequency- and region-specific neurocircuitry featured in this work may also facilitate novel treatment strategies in stroke.
Keywords: biomarker; connectivity; neuroimaging; rehabilitation; stroke.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JM reports stock holdings in Impulse Wellness LLC. FF is the lead inventory of IP filed on the topics of non-invasive brain stimulation by UNC. FF has received honoraria from the following entities in the last 12 months: Electromedical Products International, Academic Press, and Insel Spital. All other authors have no competing interests to disclose.
Figures




References
-
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63(3):272-287. - PubMed
-
- Tedesco Triccas L, Meyer S, Mantini D, et al. A systematic review investigating the relationship of electroencephalography and magnetoencephalography measurements with sensorimotor upper limb impairments after stroke. J Neurosci Methods. 2019;311:318-330. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

