CLN-617 Retains IL2 and IL12 in Injected Tumors to Drive Robust and Systemic Immune-Mediated Antitumor Activity
- PMID: 38842347
- PMCID: PMC11292205
- DOI: 10.1158/2326-6066.CIR-23-0636
CLN-617 Retains IL2 and IL12 in Injected Tumors to Drive Robust and Systemic Immune-Mediated Antitumor Activity
Abstract
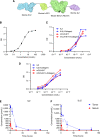
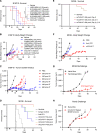
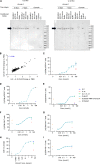
Despite clinical evidence of antitumor activity, the development of cytokine therapies has been hampered by a narrow therapeutic window and limited response rates. Two cytokines of high interest for clinical development are interleukin 2 (IL2) and interleukin 12 (IL12), which potently synergize to promote the activation and proliferation of T cells and NK cells. However, the only approved human IL2 therapy, Proleukin, is rarely used in the clinic due to systemic toxicities, and no IL12 product has been approved to date due to severe dose-limiting toxicities. Here, we describe CLN-617, a first-in-class therapeutic for intratumoral (IT) injection that co-delivers IL2 and IL12 on a single molecule in a safe and effective manner. CLN-617 is a single-chain fusion protein comprised of IL2, leukocyte-associated immunoglobulin-like receptor 2 (LAIR2), human serum albumin (HSA), and IL12. LAIR2 and HSA function to retain CLN-617 in the treated tumor by binding collagen and increasing molecular weight, respectively. We found that IT administration of a murine surrogate of CLN-617, mCLN-617, eradicated established treated and untreated tumors in syngeneic models, significantly improved response to anti-PD1 checkpoint therapy, and generated a robust abscopal response dependent on cellular immunity and antigen cross-presentation. CLN-617 is being evaluated in a clinical trial in patients with advanced solid tumors (NCT06035744).
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
N.K. Mehta reports personal fees from Cullinan Therapeutics outside the submitted work and has a pending patent for CLN-617. K. Rakhra reports other support from Cullinan Therapeutics outside the submitted work and has a pending patent for 63/579429 and for 63/593615. N. Momin reports a patent for US20230174623A1 licensed to Cullinan Therapeutics. K.D. Wittrup reports personal fees from Cullinan Therapeutics outside the submitted work and reports a patent for US20230174623A1 licenced to Cullinan Therapeutics. P.A. Baeuerle reports other support from Cullinan Oncology during the conduct of the study and personal fees and other support from Cullinan Therapeutics outside the submitted work. J.S. Michaelson reports personal fees from Cullinan Therapeutics outside the submitted work and has a pending patent for “Bi-functional linear fusion collagen-localized immunomodulatory molecules and methods thereof.” K. Rakhra, K.A. Meetze, P.A. Baeuerle, and J.S. Michaelson are employees and stock owners of Cullinan Therapeutics. N.K. Mehta and K.D. Wittrup own stock in Cullinan Therapeutics. Cullinan Therapeutics is the sponsor of an ongoing Phase I clinical trial for CLN-617. N. Momin and K.D. Wittrup report a patent US20230174623A1 licensed to Cullinan Therapeutics. N.K. Mehta, K. Rakhra, K.A. Meetze, P.A. Baeuerle and J.S. Michaelson are authors on pending patents 61/579429 and 63/593615 related to this work. No disclosures were reported by the other authors.
Figures



References
-
- Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol 2018;18:648–59. - PubMed
-
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003;3:133–46. - PubMed
-
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012;12:180–90. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

