The cost-effectiveness of procalcitonin for guiding antibiotic prescribing in individuals hospitalized with COVID-19: part of the PEACH study
- PMID: 38842487
- PMCID: PMC11290882
- DOI: 10.1093/jac/dkae167
The cost-effectiveness of procalcitonin for guiding antibiotic prescribing in individuals hospitalized with COVID-19: part of the PEACH study
Abstract
Background: Many hospitals introduced procalcitonin (PCT) testing to help diagnose bacterial coinfection in individuals with COVID-19, and guide antibiotic decision-making during the COVID-19 pandemic in the UK.
Objectives: Evaluating cost-effectiveness of using PCT to guide antibiotic decisions in individuals hospitalized with COVID-19, as part of a wider research programme.
Methods: Retrospective individual-level data on patients hospitalized with COVID-19 were collected from 11 NHS acute hospital Trusts and Health Boards from England and Wales, which varied in their use of baseline PCT testing during the first COVID-19 pandemic wave. A matched analysis (part of a wider analysis reported elsewhere) created groups of patients whose PCT was/was not tested at baseline. A model was created with combined decision tree/Markov phases, parameterized with quality-of-life/unit cost estimates from the literature, and used to estimate costs and quality-adjusted life years (QALYs). Cost-effectiveness was judged at a £20 000/QALY threshold. Uncertainty was characterized using bootstrapping.
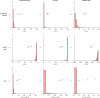
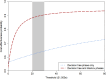
Results: People who had baseline PCT testing had shorter general ward/ICU stays and spent less time on antibiotics, though with overlap between the groups' 95% CIs. Those with baseline PCT testing accrued more QALYs (8.76 versus 8.62) and lower costs (£9830 versus £10 700). The point estimate was baseline PCT testing being dominant over no baseline testing, though with uncertainty: the probability of cost-effectiveness was 0.579 with a 1 year horizon and 0.872 with a lifetime horizon.
Conclusions: Using PCT to guide antibiotic therapy in individuals hospitalized with COVID-19 is more likely to be cost-effective than not, albeit with uncertainty.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures




References
-
- Russell CD, Fairfield CJ, Drake TMet al. . Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe 2021; 2: e354–65. 10.1016/S2666-5247(21)00090-2 - DOI - PMC - PubMed
-
- NICE . COVID-19 Rapid Guideline: Antibiotics for Pneumonia in Adults in Hospital. NICE Guideline NG173. https://www.nice.org.uk/guidance/ng173. - PubMed

