The primate cortical LFP exhibits multiple spectral and temporal gradients and widespread task dependence during visual short-term memory
- PMID: 38842507
- PMCID: PMC11383615
- DOI: 10.1152/jn.00264.2023
The primate cortical LFP exhibits multiple spectral and temporal gradients and widespread task dependence during visual short-term memory
Abstract
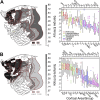
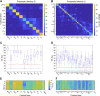
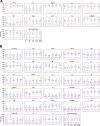
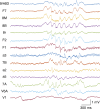
Although cognitive functions are hypothesized to be mediated by synchronous neuronal interactions in multiple frequency bands among widely distributed cortical areas, we still lack a basic understanding of the distribution and task dependence of oscillatory activity across the cortical map. Here, we ask how the spectral and temporal properties of the local field potential (LFP) vary across the primate cerebral cortex, and how they are modulated during visual short-term memory. We measured the LFP from 55 cortical areas in two macaque monkeys while they performed a visual delayed match to sample task. Analysis of peak frequencies in the LFP power spectra reveals multiple discrete frequency bands between 3 and 80 Hz that differ between the two monkeys. The LFP power in each band, as well as the sample entropy, a measure of signal complexity, display distinct spatial gradients across the cortex, some of which correlate with reported spine counts in cortical pyramidal neurons. Cortical areas can be robustly decoded using a small number of spectral and temporal parameters, and significant task-dependent increases and decreases in spectral power occur in all cortical areas. These findings reveal pronounced, widespread, and spatially organized gradients in the spectral and temporal activity of cortical areas. Task-dependent changes in cortical activity are globally distributed, even for a simple cognitive task.NEW & NOTEWORTHY We recorded extracellular electrophysiological signals from roughly the breadth and depth of a cortical hemisphere in nonhuman primates (NHPs) performing a visual memory task. Analyses of the band-limited local field potential (LFP) power displayed widespread, frequency-dependent cortical gradients in spectral power. Using a machine learning classifier, these features allowed robust cortical area decoding. Further task dependence in LFP power were found to be widespread, indicating large-scale gradients of LFP activity, and task-related activity.
Keywords: LFP; cognition; electrophysiology; large-scale; short-term memory.
Conflict of interest statement
C.M.G. is affiliated with Gray Matter Research. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures




Update of
-
The Primate Cortical LFP Exhibits Multiple Spectral and Temporal Gradients and Widespread Task-Dependence During Visual Short-Term Memory.bioRxiv [Preprint]. 2024 Jan 30:2024.01.29.577843. doi: 10.1101/2024.01.29.577843. bioRxiv. 2024. Update in: J Neurophysiol. 2024 Jul 1;132(1):206-225. doi: 10.1152/jn.00264.2023. PMID: 38352585 Free PMC article. Updated. Preprint.
Similar articles
-
The Primate Cortical LFP Exhibits Multiple Spectral and Temporal Gradients and Widespread Task-Dependence During Visual Short-Term Memory.bioRxiv [Preprint]. 2024 Jan 30:2024.01.29.577843. doi: 10.1101/2024.01.29.577843. bioRxiv. 2024. Update in: J Neurophysiol. 2024 Jul 1;132(1):206-225. doi: 10.1152/jn.00264.2023. PMID: 38352585 Free PMC article. Updated. Preprint.
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
The laminar organization of cell types in macaque cortex and its relationship to neuronal oscillations.bioRxiv [Preprint]. 2024 Mar 30:2024.03.27.587084. doi: 10.1101/2024.03.27.587084. bioRxiv. 2024. PMID: 38585801 Free PMC article. Preprint.
-
Blue-light filtering spectacle lenses for visual performance, sleep, and macular health in adults.Cochrane Database Syst Rev. 2023 Aug 18;8(8):CD013244. doi: 10.1002/14651858.CD013244.pub2. Cochrane Database Syst Rev. 2023. PMID: 37593770 Free PMC article.
-
Spectral entropy monitoring for adults and children undergoing general anaesthesia.Cochrane Database Syst Rev. 2016 Mar 14;3(3):CD010135. doi: 10.1002/14651858.CD010135.pub2. Cochrane Database Syst Rev. 2016. PMID: 26976247 Free PMC article.
Cited by
-
Large-scale interactions in predictive processing: oscillatory versus transient dynamics.Trends Cogn Sci. 2025 Feb;29(2):133-148. doi: 10.1016/j.tics.2024.09.013. Epub 2024 Oct 17. Trends Cogn Sci. 2025. PMID: 39424521 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

