Breathing new life into tissue engineering: exploring cutting-edge vascularization strategies for skin substitutes
- PMID: 38842751
- PMCID: PMC11564345
- DOI: 10.1007/s10456-024-09928-6
Breathing new life into tissue engineering: exploring cutting-edge vascularization strategies for skin substitutes
Abstract
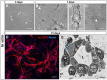
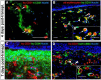
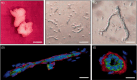
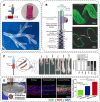
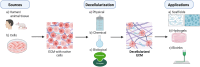
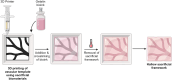
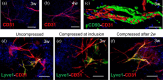
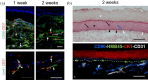
Tissue-engineered skin substitutes (TESS) emerged as a new therapeutic option to improve skin transplantation. However, establishing an adequate and rapid vascularization in TESS is a critical factor for their clinical application and successful engraftment in patients. Therefore, several methods have been applied to improve the vascularization of skin substitutes including (i) modifying the structural and physicochemical properties of dermal scaffolds; (ii) activating biological scaffolds with growth factor-releasing systems or gene vectors; and (iii) developing prevascularized skin substitutes by loading scaffolds with capillary-forming cells. This review provides a detailed overview of the most recent and important developments in the vascularization strategies for skin substitutes. On the one hand, we present cell-based approaches using stem cells, microvascular fragments, adipose tissue derived stromal vascular fraction, endothelial cells derived from blood and skin as well as other pro-angiogenic stimulation methods. On the other hand, we discuss how distinct 3D bioprinting techniques and microfluidics, miRNA manipulation, cell sheet engineering and photosynthetic scaffolds like GelMA, can enhance skin vascularization for clinical applications. Finally, we summarize and discuss the challenges and prospects of the currently available vascularization techniques that may serve as a steppingstone to a mainstream application of skin tissue engineering.
Keywords: 3D bioprinting; Angiogenesis; Blood vessels; Dermal substitutes; Endothelial cells; Mesenchymal stem cells; Scaffolds; Skin defect.
© 2024. The Author(s).
Conflict of interest statement
Figures





References
-
- Graham GP, Helmer SD, Haan JM, Khandelwal A (2013) The use of Integra® dermal regeneration template in the reconstruction of traumatic degloving injuries. J Burn Care Res 34(2):261–266. 10.1097/BCR.0b013e3182853eaf - PubMed
-
- Hosper NA, Eggink AJ, Roelofs LA, Wijnen RM, van Luyn MJ, Bank RA, Feitz WF (2010) Intra-uterine tissue engineering of full-thickness skin defects in a fetal sheep model. Biomaterials 31(14):3910–3919. 10.1016/j.biomaterials.2010.01.129 - PubMed
-
- Klar AS, Güven S, Biedermann T, Luginbühl J, Böttcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E (2014) Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 35(19):5065–5078. 10.1016/j.biomaterials.2014.02.049. (Epub 2014 Mar 27 PMID: 24680190) - PubMed
-
- Klar AS, Güven S, Zimoch J, Zapiórkowska NA, Biedermann T, Böttcher-Haberzeth S, Meuli M (2016) Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr Surg Int 32:17–27 - PubMed
Publication types
MeSH terms
Grants and funding
- European Union's Horizon 2020 research and innovation programme; SkinTerm; under the Marie Skłodowska-Curie grant agreement No 955722/Horizon 2020
- Project number: IZCOZ0_213354 (Title: "Role of CD200-CD200 receptor interactions in human diabetic skin wounds")/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
LinkOut - more resources
Full Text Sources

