Cardiovascular Events and Androgen Receptor Signaling Inhibitors in Advanced Prostate Cancer: A Systematic Review and Meta-Analysis
- PMID: 38842801
- PMCID: PMC11157448
- DOI: 10.1001/jamaoncol.2024.1549
Cardiovascular Events and Androgen Receptor Signaling Inhibitors in Advanced Prostate Cancer: A Systematic Review and Meta-Analysis
Abstract
Importance: Cardiovascular (CV) events remain a substantial cause of mortality among men with advanced and metastatic prostate cancer (PCa). The introduction of novel androgen receptor signaling inhibitors (ARSI) has transformed the treatment landscape of PCa in recent years; however, their associated CV toxic effects remains unclear.
Objective: To assess the incidence of CV events with addition of ARSI to standard of care (SOC) in locally advanced (M0) and metastatic (M1) PCa.
Data sources: Systematic searches of PubMed, Scopus, Web of Science, EMBASE, and ClinicalTrials.gov were performed from inception up to May 2023.
Study selection: Randomized clinical trials of ARSI agents (abiraterone, apalutamide, darolutamide, enzalutamide) that reported CV events among individuals with M0 and M1, hormone-sensitive prostate cancer (HSPC) and castration-resistant prostate cancer (CRPC).
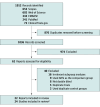
Data extraction and synthesis: A systematic review was performed in accordance with PRISMA guidance. Two authors screened and independently evaluated studies eligible for inclusion. Data extraction and bias assessment was subsequently performed.
Main outcomes and measures: A random-effects meta-analysis was performed to estimate risk ratios for the incidence of all grade and grade 3 or higher CV events (primary outcomes), in addition to hypertension, acute coronary syndrome (ACS), cardiac dysrhythmia, CV death, cerebrovascular event, and venous thromboembolism (secondary outcomes). Sources of heterogeneity were explored using meta-regression.
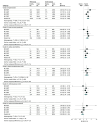
Results: There were 24 studies (n = 22 166 patients; median age range, 63-77 years; median follow-up time range, 3.9-96 months) eligible for inclusion. ARSI therapy was associated with increased risk of all grade CV event (risk ratio [RR], 1.75; 95% CI, 1.50-2.04; P < .001) and grade 3 or higher CV events (RR, 2.10; 95%, 1.72-2.55; P < .001). ARSI therapy also was associated with increased risk for grade 3 or higher events for hypertension (RR, 2.25; 95% CI, 1.74-2.90; P < .001), ACS (RR, 1.93; 95% CI, 1.43-1.60; P < .01), cardiac dysrhythmia (RR, 1.64; 95% CI, 1.23-2.17; P < .001), cerebrovascular events (RR, 1.86; 95% CI, 1.34-2.59; P < .001) and for CV-related death (RR, 2.02; 95% CI, 1.32-3.10; P = .001). Subgroup analysis demonstrated increased risk of all CV events across the disease spectrum (M0 HSPC: RR, 2.26; 95% CI, 1.36-3.75; P = .002; M1 HSPC: RR, 1.85; 95% CI, 1.47-2.31; P < .001; M0 CRPC: RR, 1.79; 95% CI, 1.13-2.81; P = .01; M1 CRPC: RR, 1.46; 95% CI, 1.16-1.83; P = .001).
Conclusions and relevance: This systematic review and meta-analysis found that the addition of ARSIs to traditional ADT was associated with increased risk of CV events across the prostate cancer disease spectrum. These results suggest that patients with prostate cancer should be advised about and monitored for the potential of increased risk of CV events with initiation of ARSI therapy alongside conventional hormonal therapy.
Conflict of interest statement
Figures


Comment in
-
Cardiovascular Risk in Prostate Cancer-A Call to Action?JAMA Oncol. 2024 Jul 1;10(7):885-886. doi: 10.1001/jamaoncol.2024.0860. JAMA Oncol. 2024. PMID: 38842826 No abstract available.
References
-
- Attard G, Murphy L, Clarke NW, et al. ; Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators . Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447-460. doi: 10.1016/S0140-6736(21)02437-5 - DOI - PMC - PubMed
-
- Attard G, Murphy L, Clarke NW, et al. ; STAMPEDE investigators . Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: final results from two randomised phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023;24(5):443-456. doi: 10.1016/S1470-2045(23)00148-1 - DOI - PMC - PubMed
-
- Parker CC, James ND, Brawley CD, et al. ; STAMPEDE Trial Collaborative Group . Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: long-term results from the STAMPEDE randomised controlled trial. PLoS Med. 2022;19(6):e1003998. doi: 10.1371/journal.pmed.1003998 - DOI - PMC - PubMed
-
- IMS Institute for Healthcare Informatics . Global oncology trend report: a review of 2015 and outlook to 2020. Published June 2016. Accessed May 7, 2024. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncolo...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

