RISING STARS: Evidence for established and emerging forms of β-cell death
- PMID: 38842911
- PMCID: PMC11285760
- DOI: 10.1530/JOE-23-0378
RISING STARS: Evidence for established and emerging forms of β-cell death
Abstract
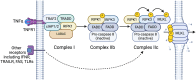
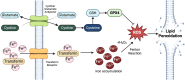
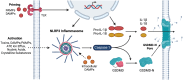
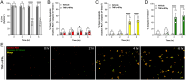
β-Cell death contributes to β-cell loss and insulin insufficiency in type 1 diabetes (T1D), and this β-cell demise has been attributed to apoptosis and necrosis. Apoptosis has been viewed as the lone form of programmed β-cell death, and evidence indicates that β-cells also undergo necrosis, regarded as an unregulated or accidental form of cell demise. More recently, studies in non-islet cell types have identified and characterized novel forms of cell death that are biochemically and morphologically distinct from apoptosis and necrosis. Several of these mechanisms of cell death have been categorized as forms of regulated necrosis and linked to inflammation and disease pathogenesis. In this review, we revisit discoveries of β-cell death in humans with diabetes and describe studies characterizing β-cell apoptosis and necrosis. We explore literature on mechanisms of regulated necrosis including necroptosis, ferroptosis and pyroptosis, review emerging literature on the significance of these mechanisms in β-cells, and discuss experimental approaches to differentiate between various mechanisms of β-cell death. Our review of the literature leads us to conclude that more detailed experimental characterization of the mechanisms of β-cell death is warranted, along with studies to better understand the impact of various forms of β-cell demise on islet inflammation and β-cell autoimmunity in pathophysiologically relevant models. Such studies will provide insight into the mechanisms of β-cell loss in T1D and may shed light on new therapeutic approaches to protect β-cells in this disease.
Keywords: diabetes; ferroptosis; inflammation; necroptosis; pyroptosis; β-cell death.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
Similar articles
-
The role of regulated necrosis in diabetes and its complications.J Mol Med (Berl). 2024 Apr;102(4):495-505. doi: 10.1007/s00109-024-02421-z. Epub 2024 Feb 23. J Mol Med (Berl). 2024. PMID: 38393662 Review.
-
Mechanisms and Therapeutic Potential of Multiple Forms of Cell Death in Myocardial Ischemia-Reperfusion Injury.Int J Mol Sci. 2024 Dec 17;25(24):13492. doi: 10.3390/ijms252413492. Int J Mol Sci. 2024. PMID: 39769255 Free PMC article. Review.
-
Partners in Crime: The Interplay of Proteins and Membranes in Regulated Necrosis.Int J Mol Sci. 2020 Mar 31;21(7):2412. doi: 10.3390/ijms21072412. Int J Mol Sci. 2020. PMID: 32244433 Free PMC article. Review.
-
Ferroptosis, necroptosis, and pyroptosis in cancer: Crucial cell death types in radiotherapy and post-radiotherapy immune activation.Radiother Oncol. 2023 Jul;184:109689. doi: 10.1016/j.radonc.2023.109689. Epub 2023 May 6. Radiother Oncol. 2023. PMID: 37150447 Review.
-
Heme Oxgenase-1, a Cardinal Modulator of Regulated Cell Death and Inflammation.Cells. 2021 Feb 28;10(3):515. doi: 10.3390/cells10030515. Cells. 2021. PMID: 33671004 Free PMC article. Review.
Cited by
-
Targeting ferroptosis: opportunities and challenges of mesenchymal stem cell therapy for type 1 diabetes mellitus.Stem Cell Res Ther. 2025 Feb 4;16(1):47. doi: 10.1186/s13287-025-04188-7. Stem Cell Res Ther. 2025. PMID: 39901210 Free PMC article. Review.
References
-
- Akirav EM Lebastchi J Galvan EM Henegariu O Akirav M Ablamunits V Lizardi PM & Herold KC. 2011Detection of β cell death in diabetes using differentially methylated circulating DNA. Proceedings of the National Academy of Sciences of the United States of America 10819018–19023. (10.1073/pnas.1111008108) - DOI - PMC - PubMed
-
- Alberts B Johnson A Lewis J Raff M Roberts K & Walter P. 2002. Programmed cell death (apoptosis). In Molecular Biology of the Cell,4th ed. New York, NY, USA: Garland Science.
-
- Anthony P.1990Robbins’ pathologic basis of disease. Journal of Clinical Pathology 43176.1–17176. (10.1136/jcp.43.2.176-a) - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

