Linking Dementia Pathology and Alteration in Brain Activation to Complex Daily Functional Decline During the Preclinical Dementia Stages: Protocol for a Prospective Observational Cohort Study
- PMID: 38842914
- PMCID: PMC11190628
- DOI: 10.2196/56726
Linking Dementia Pathology and Alteration in Brain Activation to Complex Daily Functional Decline During the Preclinical Dementia Stages: Protocol for a Prospective Observational Cohort Study
Erratum in
-
Correction: Linking Dementia Pathology and Alteration in Brain Activation to Complex Daily Functional Decline During the Preclinical Dementia Stages: Protocol for a Prospective Observational Cohort Study.JMIR Res Protoc. 2024 Oct 21;13:e66438. doi: 10.2196/66438. JMIR Res Protoc. 2024. PMID: 39432896 Free PMC article.
Abstract
Background: Progressive difficulty in performing everyday functional activities is a key diagnostic feature of dementia syndromes. However, not much is known about the neural signature of functional decline, particularly during the very early stages of dementia. Early intervention before overt impairment is observed offers the best hope of reducing the burdens of Alzheimer disease (AD) and other dementias. However, to justify early intervention, those at risk need to be detected earlier and more accurately. The decline in complex daily function (CdF) such as managing medications has been reported to precede impairment in basic activities of daily living (eg, eating and dressing).
Objective: Our goal is to establish the neural signature of decline in CdF during the preclinical dementia period.
Methods: Gait is central to many CdF and community-based activities. Hence, to elucidate the neural signature of CdF, we validated a novel electroencephalographic approach to measuring gait-related brain activation while participants perform complex gait-based functional tasks. We hypothesize that dementia-related pathology during the preclinical period activates a unique gait-related electroencephalographic (grEEG) pattern that predicts a subsequent decline in CdF.
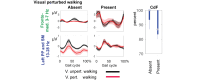
Results: We provide preliminary findings showing that older adults reporting CdF limitations can be characterized by a unique gait-related neural signature: weaker sensorimotor and stronger motor control activation. This subsample also had smaller brain volume and white matter hyperintensities in regions affected early by dementia and engaged in less physical exercise. We propose a prospective observational cohort study in cognitively unimpaired older adults with and without subclinical AD (plasma amyloid-β) and vascular (white matter hyperintensities) pathologies. We aim to (1) establish the unique grEEG activation as the neural signature and predictor of decline in CdF during the preclinical dementia period; (2) determine associations between dementia-related pathologies and incidence of the neural signature of CdF; and (3) establish associations between a dementia risk factor, physical inactivity, and the neural signature of CdF.
Conclusions: By establishing the clinical relevance and biological basis of the neural signature of CdF decline, we aim to improve prediction during the preclinical stages of ADs and other dementias. Our approach has important research and translational implications because grEEG protocols are relatively inexpensive and portable, and predicting CdF decline may have real-world benefits.
International registered report identifier (irrid): DERR1-10.2196/56726.
Keywords: EEG; electroencephalographic; mobility; preclinical dementia stages.
©Pierfilippo De Sanctis, Jeannette R Mahoney, Johanna Wagner, Helena M Blumen, Wenzhu Mowrey, Emmeline Ayers, Claudia Schneider, Natasha Orellana, Sophie Molholm, Joe Verghese. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 06.06.2024.
Conflict of interest statement
Conflicts of Interest: JRM has a financial interest in JET Worldwide Enterprises Inc, a digital health startup spun out of research conducted at the Albert Einstein College of Medicine that is not related to this project. All other authors report no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this paper apart from those disclosed.
Figures






Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Exercise for acutely hospitalised older medical patients.Cochrane Database Syst Rev. 2022 Nov 10;11(11):CD005955. doi: 10.1002/14651858.CD005955.pub3. Cochrane Database Syst Rev. 2022. PMID: 36355032 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Regional cerebral blood flow single photon emission computed tomography for detection of Frontotemporal dementia in people with suspected dementia.Cochrane Database Syst Rev. 2015 Jun 23;2015(6):CD010896. doi: 10.1002/14651858.CD010896.pub2. Cochrane Database Syst Rev. 2015. PMID: 26102272 Free PMC article.
-
Predicting cognitive decline: Deep-learning reveals subtle brain changes in pre-MCI stage.J Prev Alzheimers Dis. 2025 May;12(5):100079. doi: 10.1016/j.tjpad.2025.100079. Epub 2025 Feb 6. J Prev Alzheimers Dis. 2025. PMID: 39920001 Free PMC article.
Cited by
-
Heat shock protein 70 in Alzheimer's disease and other dementias: A possible alternative therapeutic.J Alzheimers Dis Rep. 2025 Jan 13;9:25424823241307021. doi: 10.1177/25424823241307021. eCollection 2025 Jan-Dec. J Alzheimers Dis Rep. 2025. PMID: 40034501 Free PMC article. Review.
References
-
- Verghese J, De Sanctis P, Ayers E. Everyday function profiles in prodromal stages of MCI: prospective cohort study. Alzheimers Dement. 2023;19(2):498–506. doi: 10.1002/alz.12681. https://europepmc.org/abstract/MED/35472732 - DOI - PMC - PubMed
-
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. https://linkinghub.elsevier.com/retrieve/pii/S1552-5260(18)30072-4 S1552-5260(18)30072-4 - DOI - PMC - PubMed
-
- Dubbelman MA, Sanchez J, Schultz AP, Rentz DM, Amariglio RE, Sikkes SAM, Sperling RA, Johnson KA, Marshall GA. Everyday functioning and entorhinal and inferior temporal tau burden in cognitively normal older adults. J Prev Alzheimers Dis. 2022;9(4):801–808. doi: 10.14283/jpad.2022.58. https://europepmc.org/abstract/MED/36281685 - DOI - PMC - PubMed
-
- Dubbelman MA, Jutten RJ, Tomaszewski Farias SE, Amariglio RE, Buckley RF, Visser PJ, Rentz DM, Johnson KA, Properzi MJ, Schultz A, Donovan N, Gatchell JR, Teunissen CE, Van Berckel BNM, Van der Flier WM, Sperling RA, Papp KV, Scheltens P, Marshall GA, Sikkes SAM. Decline in cognitively complex everyday activities accelerates along the Alzheimer's disease continuum. Alzheimers Res Ther. 2020;12(1):138. doi: 10.1186/s13195-020-00706-2. https://alzres.biomedcentral.com/articles/10.1186/s13195-020-00706-2 10.1186/s13195-020-00706-2 - DOI - DOI - PMC - PubMed
-
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. https://europepmc.org/abstract/MED/22487856 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

