Two weeks of acarbose treatment shows no effect on gut microbiome composition in patients with type 2 diabetes: a randomised, placebo-controlled, double-blind, crossover study
- PMID: 38842918
- PMCID: PMC11227053
- DOI: 10.1530/EC-24-0052
Two weeks of acarbose treatment shows no effect on gut microbiome composition in patients with type 2 diabetes: a randomised, placebo-controlled, double-blind, crossover study
Abstract
Aim: The alpha-glucosidase inhibitor acarbose is approved for the treatment of type 2 diabetes (T2D). It acts in the lumen of the gut by reducing intestinal hydrolysis and absorption of ingested carbohydrates. This reduces postprandial blood glucose concentration and increases the content of carbohydrates in the distal parts of the intestine potentially influencing gut microbiome (GM) composition and possibly impacting the gut microbiome (GM) dysbiosis associated with T2D. Here, we investigated the effect of acarbose on GM composition in patients with T2D.
Methods: Faecal samples were collected in a previously conducted randomised, placebo-controlled, double-blind, crossover study in which 15 individuals with metformin-treated T2D (age 57-85 years, HbA1c 40-74 mmol/mol, BMI 23.6-34.6 kg/m2) were subjected to two 14-day treatment periods with acarbose and placebo, respectively, separated by a 6-week wash-out period. Faecal samples were collected before and by the end of each treatment period. The GM profiles were evaluated by 16S rRNA gene amplicon sequencing.
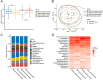
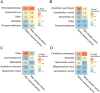
Results: The GM profiles after the treatment periods with acarbose or placebo remained unaffected (P > 0.7) when compared with the GM profiles before treatment. This applied to the analysis of within-sample diversity (α-diversity) and between-sample bacterial composition diversity (β-diversity). Additionally, no dominant bacterial species differentiated the treatment groups, and only minor increases in the relative abundances of Klebsiella spp. and Escherichia coli (P < 0.05) were observed after acarbose treatment.
Conclusion: In patients with metformin-treated T2D, 14 days of treatment with acarbose showed only minor effects on GM as seen in increased relative abundances of Klebsiella spp. and Escherichia coli.
Keywords: acarbose; alpha-glucosidase inhibitor; gut bacteria; gut microbiome; type 2 diabetes.
Conflict of interest statement
NBD, LSG, LSH, and TSR report no conflict of interest that could be perceived as prejudicing the impartiality of the study reported. FKK has served on scientific advisory panels and/or been part of speaker’s bureaus for, served as a consultant to, and/or received research support from 89bio, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Pharmacosmos, Sanofi, Structure Therapeutics, and Zealand Pharma.
Figures
Similar articles
-
Acarbose diminishes postprandial suppression of bone resorption in patients with type 2 diabetes.Bone. 2023 May;170:116687. doi: 10.1016/j.bone.2023.116687. Epub 2023 Feb 6. Bone. 2023. PMID: 36754130 Clinical Trial.
-
The role of GLP-1 in the postprandial effects of acarbose in type 2 diabetes.Eur J Endocrinol. 2021 Mar;184(3):383-394. doi: 10.1530/EJE-20-1121. Eur J Endocrinol. 2021. PMID: 33449919 Clinical Trial.
-
Metagenomic analysis reveals crosstalk between gut microbiota and glucose-lowering drugs targeting the gastrointestinal tract in Chinese patients with type 2 diabetes: a 6 month, two-arm randomised trial.Diabetologia. 2022 Oct;65(10):1613-1626. doi: 10.1007/s00125-022-05768-5. Epub 2022 Aug 5. Diabetologia. 2022. PMID: 35930018 Free PMC article. Clinical Trial.
-
Effects of Non-insulin Anti-hyperglycemic Agents on Gut Microbiota: A Systematic Review on Human and Animal Studies.Front Endocrinol (Lausanne). 2020 Sep 23;11:573891. doi: 10.3389/fendo.2020.573891. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33071980 Free PMC article.
-
Is there a role for alpha-glucosidase inhibitors in the prevention of type 2 diabetes mellitus?Drugs. 2003;63(10):933-51. doi: 10.2165/00003495-200363100-00002. Drugs. 2003. PMID: 12699398 Review.
References
-
- Ruuskanen MO, Erawijantari PP, Havulinna AS, Liu Y, Méric G, Tuomilehto J, Inouye M, Jousilahti P, Salomaa V, Jain M, et al.Gut microbiome composition is predictive of incident type 2 diabetes in a population cohort of 5572 finnish adults. Diabetes Care 202245811–818. (10.2337/dc21-2358) - DOI - PMC - PubMed
-
- Food US and Administration Drug. PRECOSE® (acarbose tablets). Silver Spring, MD, USA: FDA, 2011. (available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020482s024lbl.pdf)
LinkOut - more resources
Full Text Sources
Miscellaneous

